Recombinant galactose agglutinin-1 two-string protein and preparation method thereof
A galectin and protein technology, applied in the biological field, can solve problems such as tumor growth inhibition, and achieve the effect of simple separation and purification process and easy to expand production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
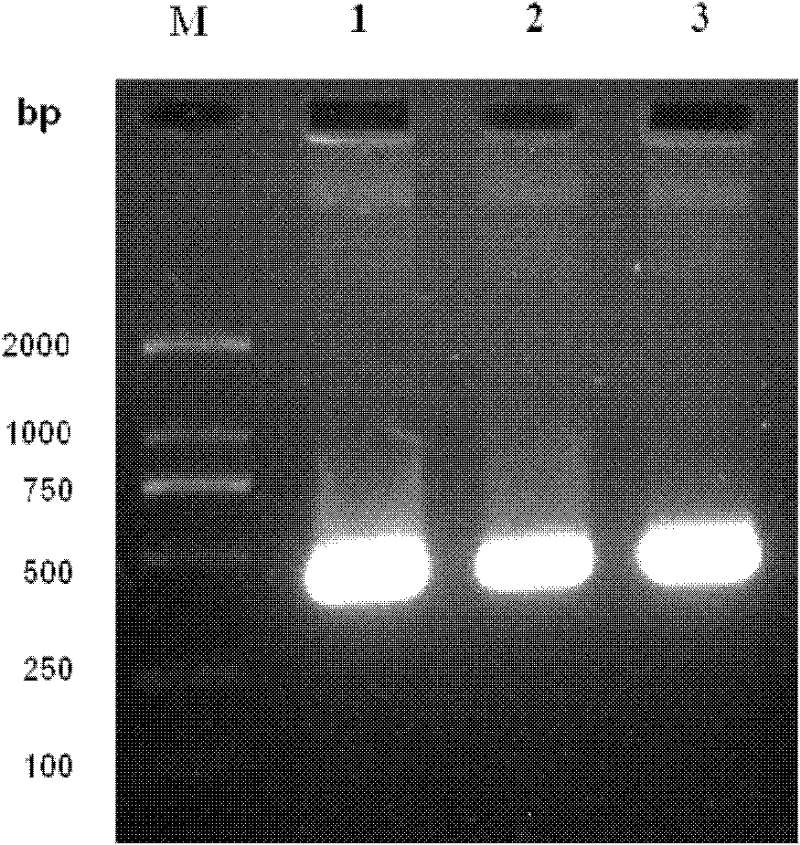
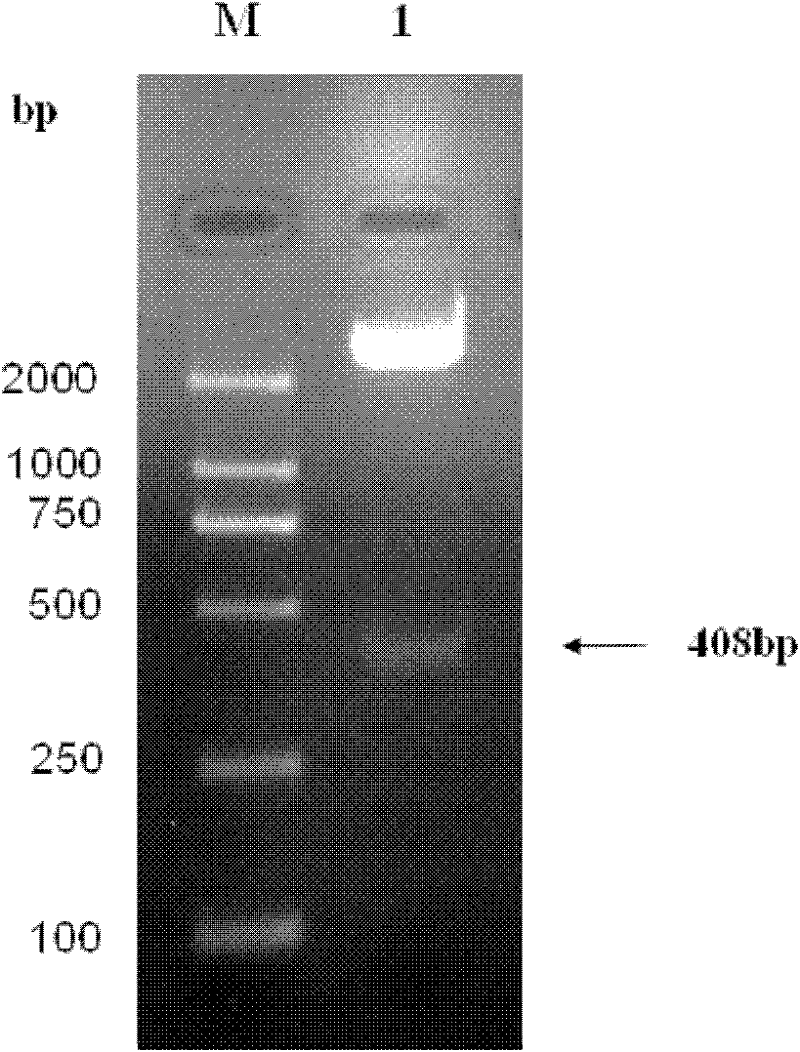
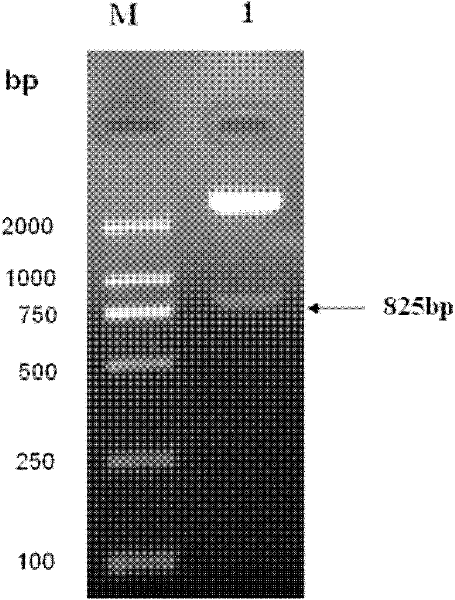
[0045] The present invention provides biologically active recombinant Galectin-1 protein through biotechnology methods, and constructs prokaryotic expression vectors pET-22b(+)-Gal-1 and pET-22b(+)-Gal-1②, respectively transfecting BL21 construction projects Bacteria, and then induced expression by IPTG, chromatographic separation and purification after lysing to obtain recombinant Galectin-1 monomer protein or recombinant Galectin-1 binary protein with biological activity. The erythrocyte agglutination test confirmed that the two recombinant Galectin-1 proteins had obvious biological activities, and the biological activity of the recombinant Galectin-1 dimer protein was higher than that of the recombinant Galectin-1 monomeric protein. The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments, which are explanations of the present invention rather than limitations.
[0046] 1. Cloning of Galectin-1 monomer gen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com