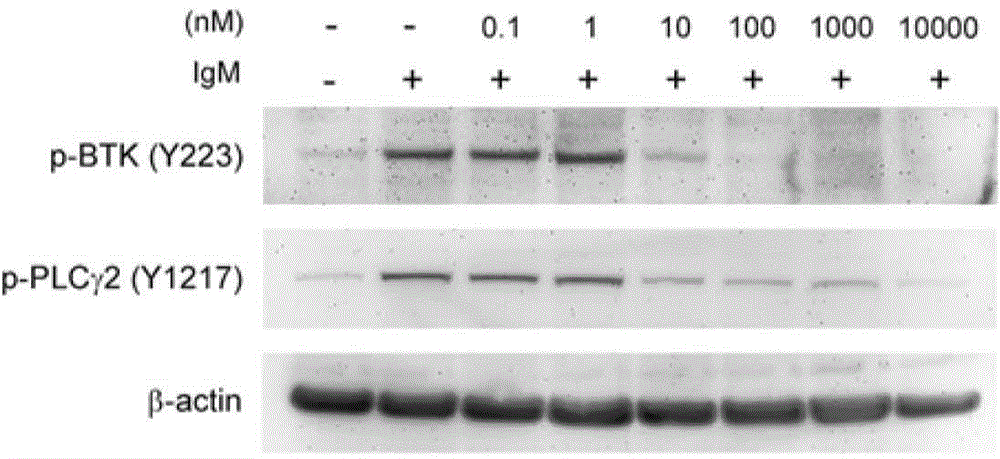
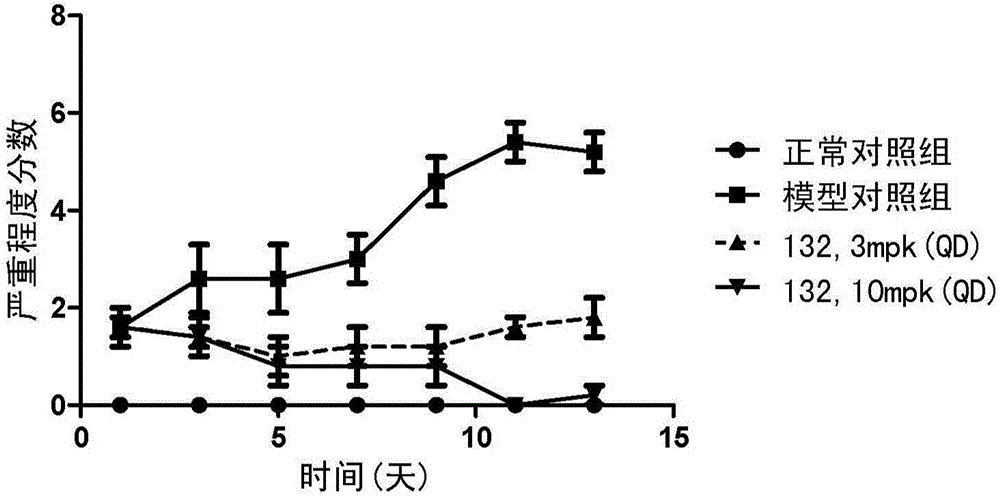
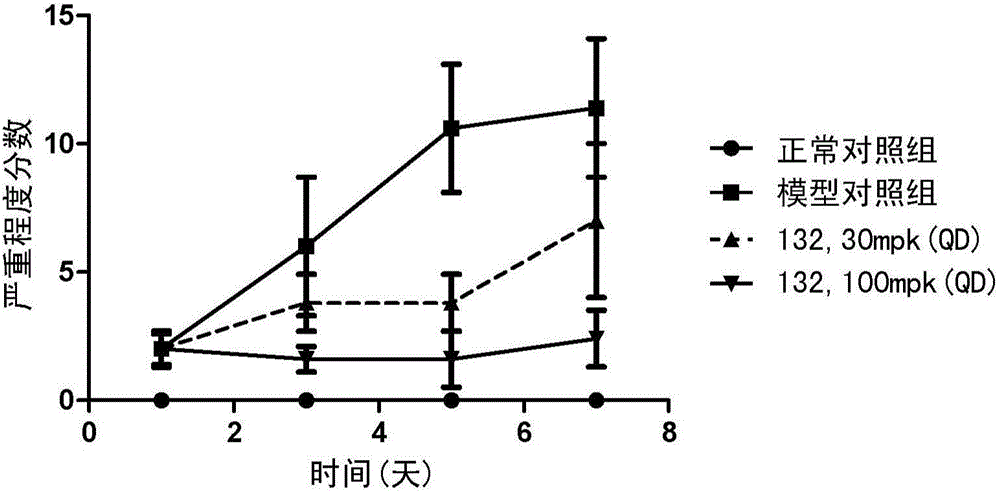
Compound for inhibiting activity of BTK and/or JAK3
A compound and solvate technology, applied in the field of compounds that inhibit BTK and/or JAK3 kinase activity, can solve problems such as immunodeficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0405] Preparation of compounds of the present invention
[0406] The following reaction scheme exemplifies the preparation method of the compound of formula I of the present invention by taking compounds of formula Ia, formula Ib and formula Ic as examples.
[0407] It should be understood by those skilled in the art that in the following description, combinations of substituents are permissible only when such combination can result in a stable compound.
[0408] Those skilled in the art will also understand that in the methods described below, intermediate compound functional groups may need to be protected by an appropriate protecting group "PG". Such functional groups include hydroxyl, amino, mercapto and carboxylic acid. Suitable hydroxy protecting groups include trialkylsilyl or diarylalkylsilyl groups (eg tert-butyldimethylsilyl, tert-butyldiphenylsilyl or trimethylsilyl) , tetrahydropyranyl, benzyl, etc. Suitable protecting groups for amino, amidino and guanidino ...
Embodiment 1
[0466] N-(cis-4-((2-((1-methyl-1H-pyrazol-4-yl)amino)thiophene[3,2-d]pyrimidin-4-yl)amino)cyclohexyl)propene Preparation of amides
[0467]
[0468] Step 1: Preparation of tert-butyl cis-4-((2-chlorothien[3,2-d]pyrimidin-4-yl)amino)cyclohexylaminocarboxylate
[0469]
[0470] 2,4-Dichlorothieno[3,2-d]pyrimidine (678 mg, 3.31 mmol) and tert-butyl cis-4-aminocyclohexylaminocarboxylate (849 mg, 3.97 mmol) were dissolved in tetrahydrofuran (10 mL), N,N-Diisopropylethylamine (0.9 mL, 4.97 mmol) was added. The reaction solution was reacted at 70° C. overnight, and then concentrated. The residue was added to water (20 mL), extracted with ethyl acetate (20 mL*3). The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: n-hexane: ethyl acetate = 1:5) to obtain 1.07 g of a white solid. Yield: 84.7%. MS(ESI,m / z):[M+H] + :383.4; 1 H-NMR (300MHz, CDCl 3 )δ:7.74(d,1H,J=5.4Hz),7.35...
Embodiment 57
[0496] N-(cis-4-((2-((1-methyl-1H-pyrazol-4-yl)amino)furo[3,2-d]pyrimidin-4-yl)amino)cyclohexyl)propene Preparation of amides
[0497]
[0498] Step 1: cis-N 1 Preparation of -(2-chlorofuro[3,2-d]pyrimidin-4-yl)cyclohexyl-1,4-diamine
[0499]
[0500] Dissolve 2,4-dichlorofuro[3,2-d]pyrimidine (188mg, 1mmol) in dichloromethane (5mL), add dropwise to cis-1,4-cyclohexanediamine (456mg, 4.0mmol) solution in dichloromethane (10 mL). The reaction solution was stirred at room temperature overnight, then concentrated. The residue was added to water (20 mL), extracted with ethyl acetate (20 mL*3). The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by silica gel column chromatography (eluent: n-hexane: ethyl acetate = 1:1) to obtain 140 mg of a light yellow solid. Yield: 52.6%. MS(ESI,m / z):[M+H] + :267.1; 1 H-NMR (300MHz, CD 3 OD):8.01-8.00(d,1H,J=2.1Hz),6.78-6.77(d,1H,J=2.1Hz),4.24-2.22(m,1H),3.07-3.03(m,1H),1.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com