Solvent-substitution solvent used in aerogel production, and hydrophobised aerogel production method using same
A technology for replacing solvents and aerogels, applied in chemical instruments and methods, silica, inorganic chemistry, etc., can solve the problems of low quality of butanol solvent, butanol loss, etc. , the effect of high porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
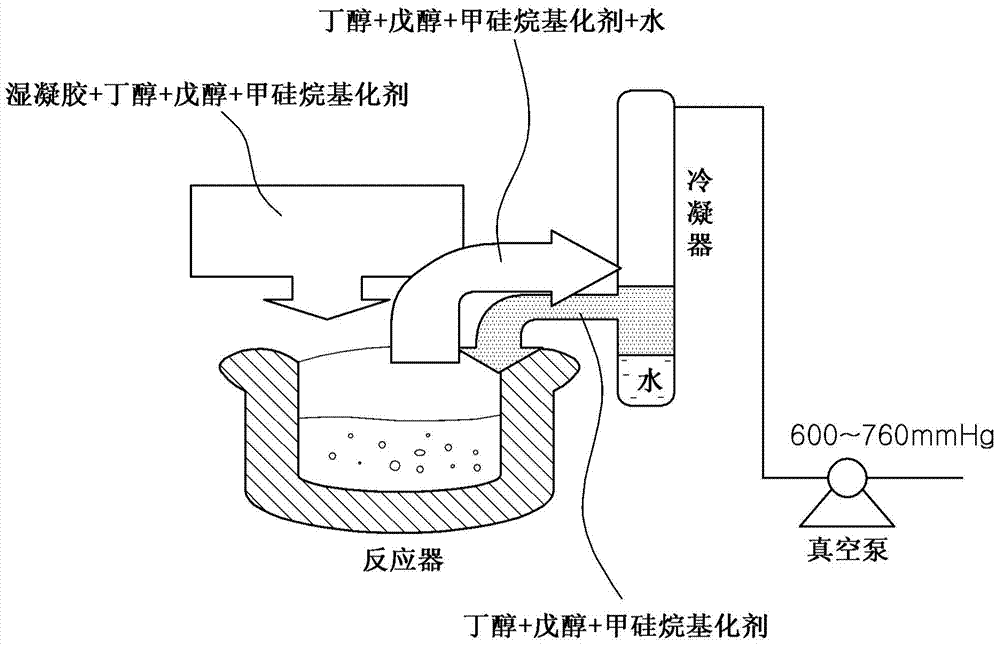
[0114] 25% by weight of water glass (sodium silicate) was added to 1 L of 2N hydrochloric acid at room temperature until pH 4 was obtained, and due to the reaction between sodium silicate and hydrochloric acid, wet gel beads were formed. The wet gel beads were washed with distilled water and filtered to remove impurities. 300 g of wet gel beads were inserted into the reactor, and 300 g of n-pentanol (an industrial product with a purity of 90% by weight to 95% by weight) was mixed with the wet gel beads. Then, by the reflux distillation method, by using a heating mantle to heat the reactor until the liquid boils, the stratified water is discharged through the lower valve, while the vapor rising from the boiling liquid is condensed using a condenser, and the reaction liquid mixture (solvent-displacing solvent ) was introduced back into the reactor (if the pressure drops to 30 mmHg, it takes about 40 minutes), and the mixture was allowed to react at atmospheric pressure for 5 hou...
Embodiment 2
[0116] 25% by weight of water glass (sodium silicate) was added to 1 L of 2N hydrochloric acid at room temperature until pH 4 was obtained, and due to the reaction between sodium silicate and hydrochloric acid, wet gel beads were formed. The wet gel beads were washed with distilled water and filtered to remove impurities. Separately, about 3500 g of n-pentanol (an amount for 7 uses) was prepared. 300 g of silica wet gel beads were added to 440 g of n-amyl alcohol (solvent), and the resulting mixture was inserted into a mixer and stirred at room temperature at about 7500 rpm for about 5 minutes. After this time, the mixture was filtered and the water layered down in the filtrate was removed. Then, the upwardly layered solvent in the filtrate was stored separately. The solid phase silica material remaining after filtration was mixed with 440 g of n-amyl alcohol solvent and stirred in a mixer at about 7500 rpm for about 15 minutes. Then, the resulting mixture was filtered to o...
Embodiment 3
[0118]25% by weight of water glass (sodium silicate) was added to 2 L of 1N hydrochloric acid at room temperature until pH 6 was obtained, and due to the reaction between sodium silicate and hydrochloric acid, wet gel beads were formed. The wet gel beads were washed with distilled water and filtered to remove impurities. 300 g of wet gel powder was inserted into the reactor, and 300 g of n-amyl alcohol (an industrial product with a purity of 90% by weight to 95% by weight) was mixed with the wet gel powder. Then, by the reflux distillation method, by using a heating mantle to heat the reactor until the liquid boils, the stratified water is discharged through the lower valve, while the vapor rising from the boiling liquid is condensed using a condenser, and the reaction liquid mixture (solvent-displacing solvent ) was introduced back into the reactor (if the pressure drops to 30 mmHg, it takes about 40 minutes), and the mixture was allowed to react at atmospheric pressure for 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com