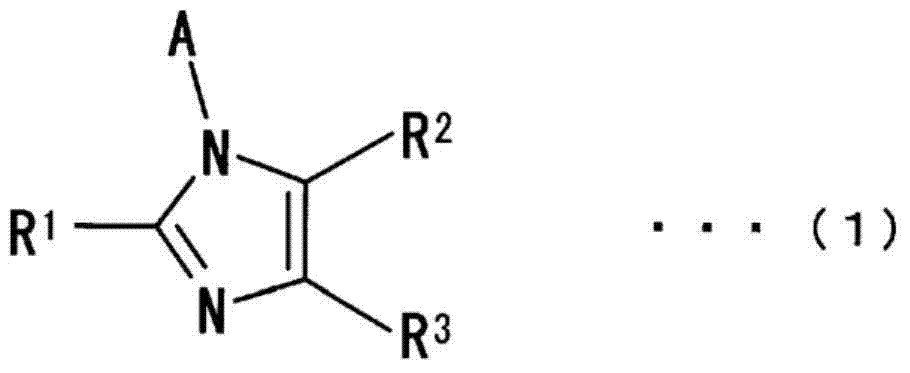
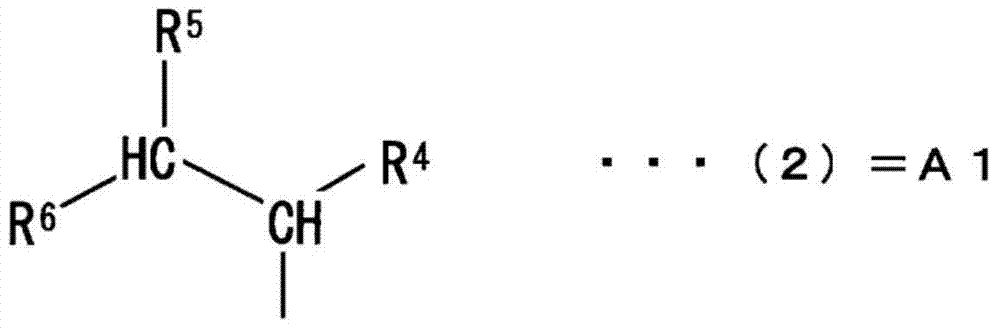
Curing agent for anionically curable compounds, curable composition, cured product, novel imidazole-based compound and use of same
An imidazole-based compound, curing technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of imidazole-based curing agent, exhaust, etc., to achieve excellent uniform mixing, high storage stability, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
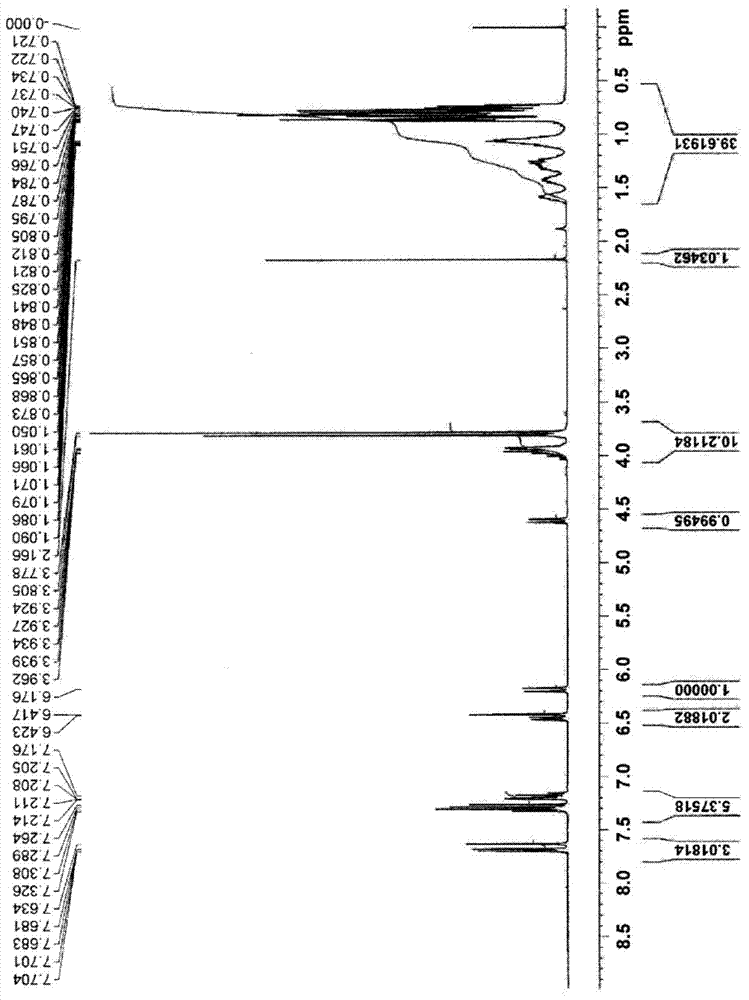
[0079] Hereinafter, although an Example is given and this invention is demonstrated more concretely, this invention is not limited to the following Example unless the summary is exceeded.
Synthetic example 1
[0080] [Synthesis Example 1: Synthesis of Dimethyl 2-(2-butylimidazol-1-yl)succinate]
[0081] 9.5 g (0.06 mol) of diazabicycloundecene (DBU), 18 mL of acetonitrile, and 17.1 g (0.14 mol) of 2-butylimidazole were put into a 100 mL four-necked flask, and stirred at 25°C. 18.0 g (0.12 mol) of dimethyl fumarate was dripped there, and it was made to react at 25 degreeC for 3 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, followed by extraction with 70 mL of dichloromethane and 50 mL of water. The fractionated organic layer was concentrated, and the obtained concentrate was purified by silica gel column chromatography (ethyl acetate / hexane=1 / 1) to obtain liquid 2-(2-butylimidazol-1-yl)succinic acid Dimethyl ester. The obtained dimethyl 2-(2-butylimidazol-1-yl)succinate was 13.1 g, and the yield was 39%.
[0082] 2-(2-butylimidazol-1-yl) dimethyl succinate begins the detachment reaction of the protecting group at a temperature of 17...
Synthetic example 2
[0083] [Synthesis Example 2: Synthesis of Dimethyl 2-(2-Undecylimidazol-1-yl)succinate]
[0084] 7.9 g (0.05 mol) of DBU, 15 mL of acetonitrile, and 25.5 g (0.11 mol) of 2-undecylimidazole were charged into a 100 mL four-necked flask, and stirred at 25°C. 15.0 g (0.10 mol) of dimethyl fumarate was dripped there, and it was made to react at 25 degreeC for 3 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, followed by extraction with 70 mL of dichloromethane and 50 mL of water. The fractionated organic layer was concentrated, and the obtained concentrate was purified by silica gel column chromatography (ethyl acetate / hexane=3 / 2) to obtain liquid 2-(2-undecylimidazol-1-yl) Dimethyl succinate. The obtained dimethyl 2-(2-undecylimidazol-1-yl)succinate was 12.1 g, and the yield was 32%.
[0085] 2-(2-undecylimidazol-1-yl) dimethyl succinate begins the detachment reaction of the protecting group at a temperature of 194°C, 2-(2-undecyli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com