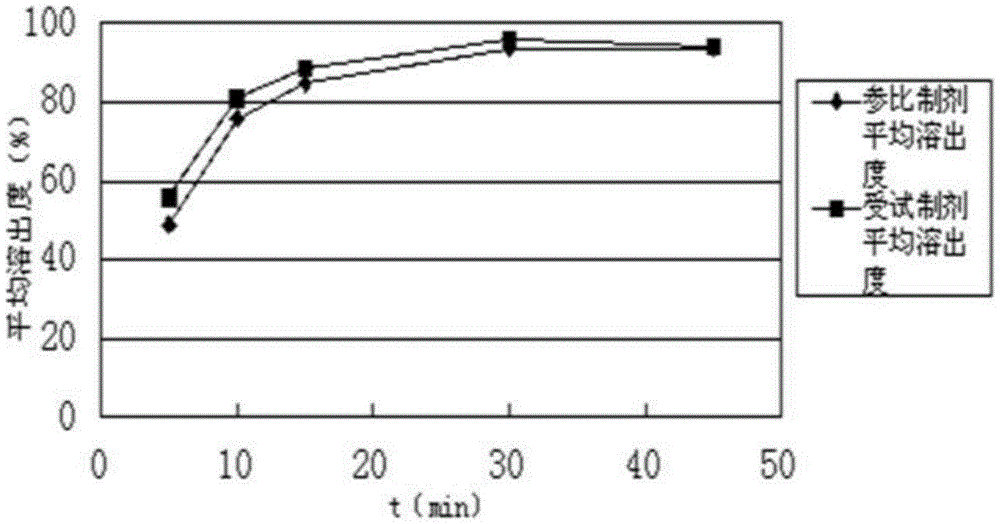
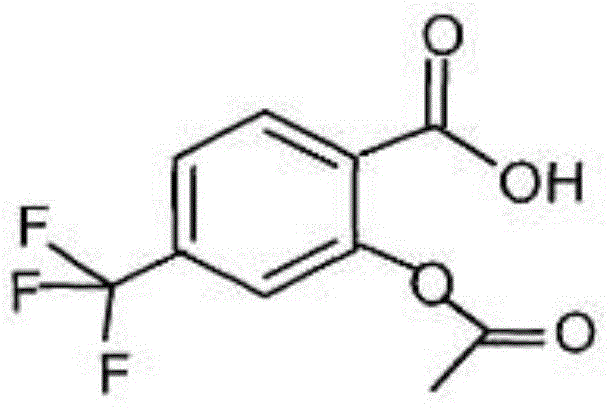
A kind of triflusalol pharmaceutical composition with high bioavailability
A technology of availability and trifluxalol, which is applied in the field of trifluxalon pharmaceutical compositions, can solve the problems of low bioavailability and non-bioequivalence, and achieve the effect of high bioavailability and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, triflusal capsules
[0055] prescription:
[0056]
[0057] Preparation Process:
[0058] 1) Preparation of adhesive: Take 15g of hypromellose, add appropriate amount of 80% ethanol solution, stir to dissolve, and use 80% ethanol solution to make up to 1000ml;
[0059] 2) Grind triflusalox through a 120-mesh sieve, and crush lactose, tartaric acid, and sodium carboxymethyl starch through a 100-mesh sieve respectively, weigh the prescription amount of trifluxal, lactose, tartaric acid, and sodium starch glycolate and mix well ;
[0060] 3) Add an appropriate amount of binder to make a soft material; granulate with a 24-mesh sieve, and ventilate and dry at 60°C;
[0061] 4) Add the prescription amount of talcum powder and mix, use a 22-mesh sieve to granulate, and mix evenly to get the product;
[0062] 5) Measure the main drug content of the granules;
[0063] 6) Pack capsules and polish;
[0064] 7) Packaging.
Embodiment 2
[0065] Embodiment 2, triflusalol tablet
[0066] prescription:
[0067]
[0068] Preparation Process:
[0069] 1) Preparation of adhesive: Take 15g of hypromellose, add appropriate amount of 80% ethanol solution, stir to dissolve, and use 80% ethanol solution to make up to 1000ml;
[0070] 2) Grind triflusalox through a 120-mesh sieve, and crush lactose, tartaric acid, and sodium starch glycolate through a 100-mesh sieve respectively, and weigh the prescribed amount of triflusal, lactose, tartaric acid and 2 / 3 of the prescribed amount of carboxymethyl starch Starch sodium is fully mixed evenly;
[0071] 3) Add an appropriate amount of binder to make a soft material; granulate with a 16-mesh sieve, and ventilate and dry at 60°C;
[0072] 4) Add the remaining 1 / 3 amount of sodium carboxymethyl starch and talcum powder to mix, use a 14-mesh sieve to sieve the granules, and mix well to get the product;
[0073] 5) Measure the content, determine the tablet weight, and compress...
Embodiment 3
[0076] Embodiment 3, triflusal capsules
[0077] prescription:
[0078]
[0079] Preparation method: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com