Biodegradable medical packaging material and preparation method thereof
A packaging material and medical technology, applied in the field of degradable medical packaging materials and their preparation, can solve the problems of animal death, difficult to degrade by natural microorganisms, environmental hazards, etc., and achieve excellent degradation performance, good physical performance and degradable performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
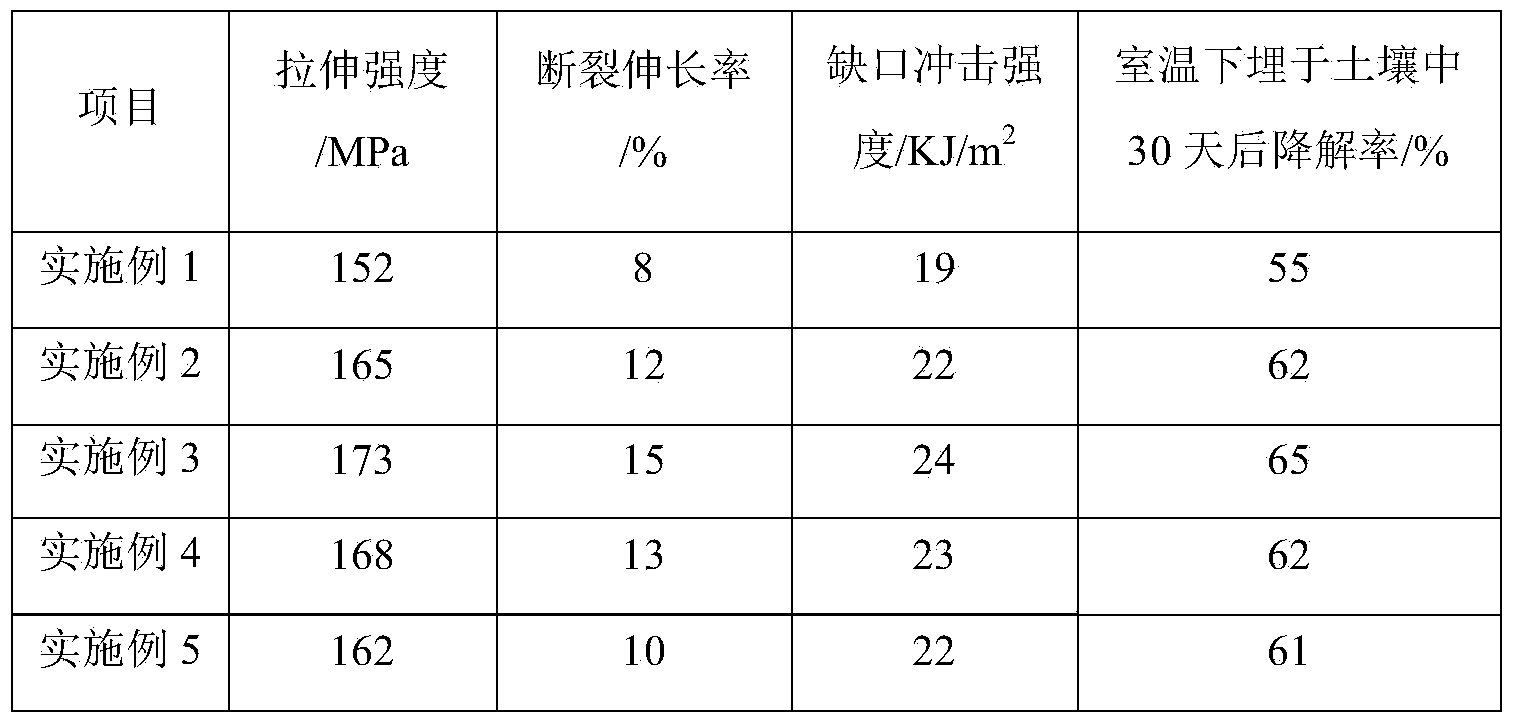
Embodiment 1
[0018] A degradable medical packaging material, comprising by weight components: 20 parts of PE, 30 parts of PVC, 10760.5 parts of antioxidant, 0.3 parts of auxiliary antioxidant GW5080, 1 part of ethylenediamine, 3 parts of polyester fiber, 5 parts of gelatin, 5 parts of lecithin, 2 parts of polypropylene, 3 parts of polylactic acid, 3 parts of sodium starch, 2 parts of glycerin, 0.5 parts of emulsified silicone oil, 3 parts of succinic acid, and 5 parts of chitosan.
[0019] The preparation method of described degradable medical packaging material, comprises the following steps:
[0020] Step 1: Add PE, PVC, ethylenediamine, polyester fiber, gelatin, lecithin, polypropylene, sodium starch and chitosan into the reaction kettle, at a temperature of 80°C and a vacuum of 0.05MPa , stirred and reacted for 40 minutes, and the stirring speed was 90 rpm to obtain mixture 1;
[0021] Step 2, adding the remaining components to the mixture 1 obtained in step 1, stirring and mixing eve...
Embodiment 2
[0024] A degradable medical packaging material, comprising by weight components: 25 parts of PE, 33 parts of PVC, 0.8 parts of antioxidant 1076, 0.4 parts of auxiliary antioxidant GW508, 3 parts of ethylenediamine, 5 parts of polyester fiber, 6 parts of gelatin, 7 parts of lecithin, 5 parts of polypropylene, 4 parts of polylactic acid, 5 parts of sodium starch, 3 parts of glycerin, 0.8 parts of emulsified silicone oil, 4 parts of succinic acid, and 7 parts of chitosan.
[0025] The preparation method of described degradable medical packaging material, comprises the following steps:
[0026] Step 1: Add PE, PVC, ethylenediamine, polyester fiber, gelatin, lecithin, polypropylene, sodium starch and chitosan into the reaction kettle at a temperature of 85°C and a vacuum of 0.03MPa , stirred and reacted for 42 minutes, and the stirring speed was 95 rpm to obtain mixture 1;
[0027] Step 2, adding the remaining components into the mixture 1 obtained in the step 1, stirring and mixi...
Embodiment 3
[0030] A degradable medical packaging material, comprising by weight components: 27 parts of PE, 35 parts of PVC, 1 part of antioxidant 1076, 0.6 part of auxiliary antioxidant GW508, 4 parts of ethylenediamine, 6 parts of polyester fiber, 7 parts of gelatin, 8 parts of lecithin, 6 parts of polypropylene, 5 parts of polylactic acid, 6 parts of sodium starch, 4 parts of glycerin, 1.2 parts of emulsified silicone oil, 6 parts of succinic acid, and 8 parts of chitosan.
[0031] The preparation method of described degradable medical packaging material, comprises the following steps:
[0032] Step 1: Add PE, PVC, ethylenediamine, polyester fiber, gelatin, lecithin, polypropylene, sodium starch and chitosan into the reaction kettle at a temperature of 86°C and a vacuum of 0.03MPa , stirred and reacted for 50 minutes, and the stirring speed was 110 rpm to obtain mixture 1;
[0033] Step 2, adding the remaining components to the mixture 1 obtained in the step 1, stirring and mixing ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Notched impact strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com