Elastin-like polypeptides (ELP) for prokaryotic expression of fusion protein Prx by non-digestion and non-chromatography purifying method
An elastin-like and fusion protein technology, applied in the field of genetic engineering, to achieve the effect of improving elastic function, simple operation and reducing impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] combined with figure 1 Describe the method of preparation.
[0039] 1. Construction of promoter protein, precursor protein expression vector and host cell
[0040] (a) Synthesis of ELP-IN, ELP-IC and PrxI genes
[0041] The primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the DNAs of ELP-IN and ELP-IC (SEQ ID NO: 1 and SEQ ID NO: 3) were obtained by PCR. The primer sequences are as follows:
[0042] ELP-IN:
[0043] 5' primer:
[0044] 5'GG CCATGG CCGTGCCTGGTAAAGGAGTTCC3' (Nco Ⅰ)
[0045] 3' primer:
[0046] 5'GCGC AAGCTT ATTCTTTCACATACAGGCACATGCC3'(HindⅢ)
[0047] ELP-IC:
[0048] 5' primer:
[0049] 5'CC CATATG GTGCCTGGTAAAGGAG3' (NdeI)
[0050] 3' primer:
[0051] 5'TTAAGGATCCGCTGTTATGGGTCAGAATATCGTT3'(BamH Ⅰ)
[0052] In addition, two primers were designed and synthesized for PCR cloning the cDNA of Prx Ⅰ. The primer sequences are as follows:
[0053] 5' primer:
[0054] 5'CG GGATCC ATGTCTTCAGGAAATG...
Embodiment 2
[0072] The groping of the indirect condition of embodiment 2 object protein
[0073] (a) Incubate for 4 hours under different temperature conditions
[0074] After the purified protein was incubated at different temperatures (4°C, 10°C, 15°C, 20°C, 25°C) for different durations (10min, 20min, 30min, 1h, 2h, 4h) at a ratio of 1:1, SDS- PAGE electrophoresis detection, drawing the relationship curve of self-cutting percentage and time under different temperature conditions ( Figure 7 ). Protein self-cleavage is relatively complete at 25°C. In order to reduce the impact of high temperature on the activity of the target protein, 25°C is temporarily used to induce self-cleavage.
[0075]
[0076] 1×Reaction buffer: (50mM Tris, 100mM NaCl, 5% Glycerol)
[0077] (b) 25°C water bath induces protein splicing
[0078] The promoter protein and the precursor protein were mixed at a ratio of 1:1, and the self-cleavage was induced for different periods of time (1min, 5min, 10min, 0.5...
Embodiment 3
[0079] Purification and activity detection of embodiment 3 target protein
[0080] (a) Purification of target protein
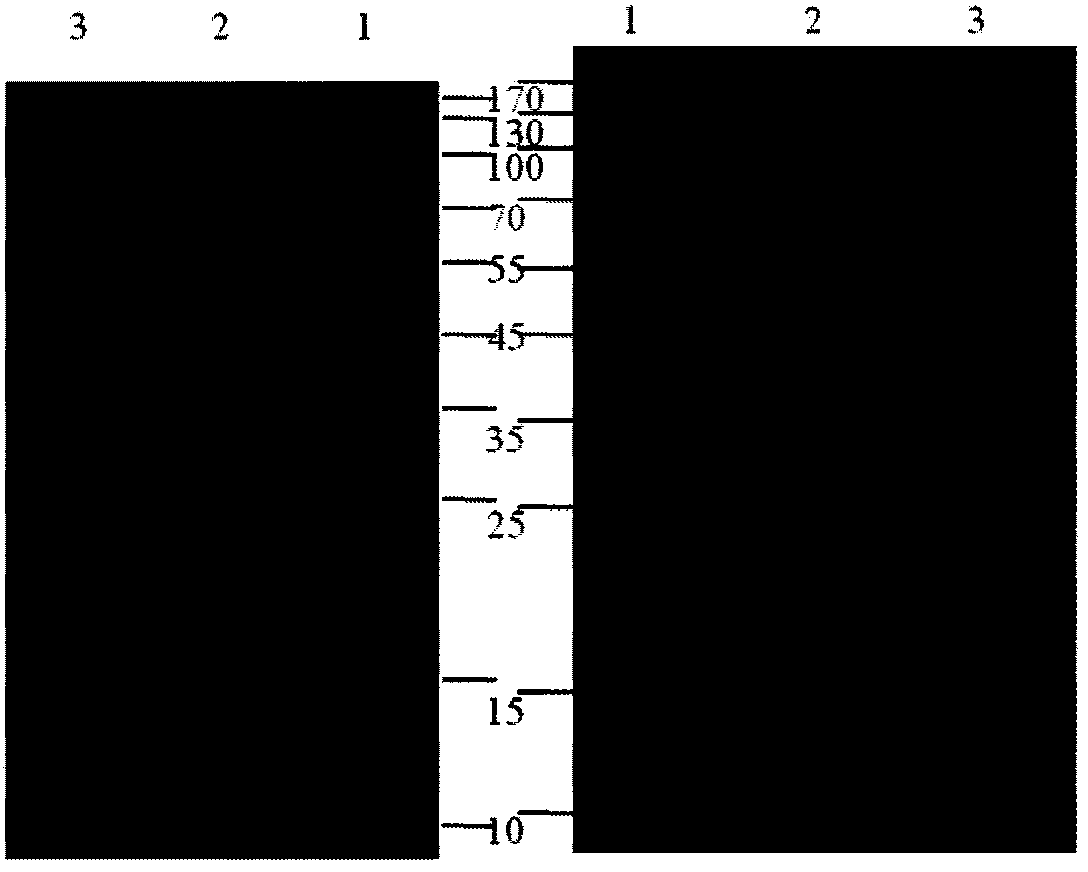
[0081] According to the system of Example 2, the purified protein was induced to self-cleavage of the precursor protein for 0.5 h at an optimum temperature of 25° C., and then purified by the ITC method to obtain the target protein PrxI ( Figure 9 ).
[0082] (b) Activity detection of PrxI
[0083] The activity of the PrxI prepared above was determined by referring to the PrxI activity detection method mentioned in the literature Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemia cells. Detect the A340 value at different time points with BioTek Synergy2 multifunctional microplate reader (Molecular Devices, USA), draw the curve of A340 value changing with time ( Figure 10 ).
[0084] Results: The activity of the sample was comparable to that of the standard, and the whole purification process did not affect the activity of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com