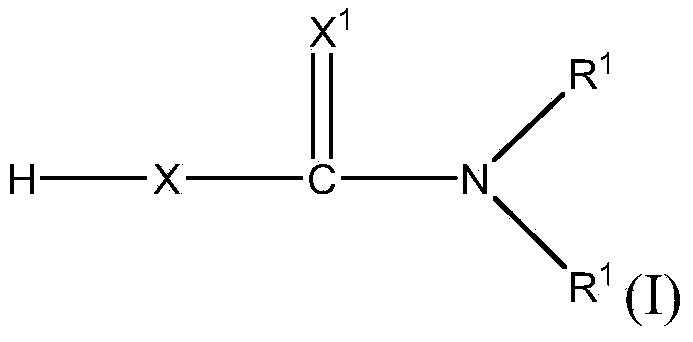
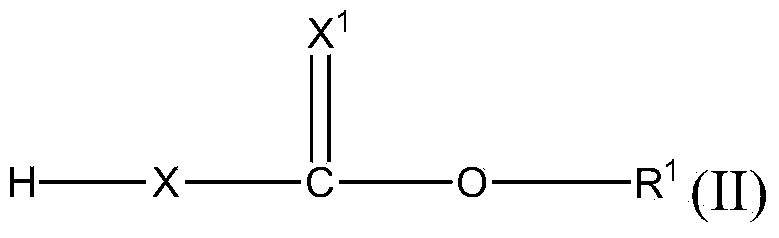
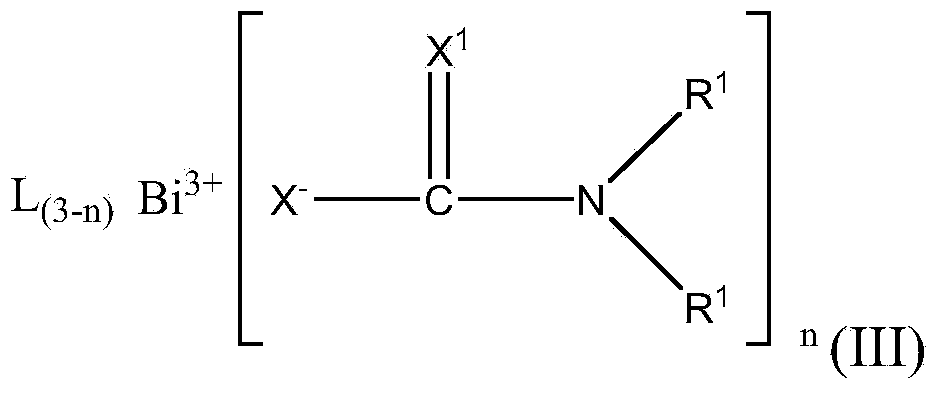
Polyurethanes made using bismuth thiocarbamate or thiocarbonate salts as catalysts
A dithiocarbonate and catalyst technology, applied in the field of polymer preparation, can solve the problems of short storage period, high reactivity of bismuth catalyst, loss of activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0093] Examples 1 and 2 and comparative samples A and B
[0094] The catalyst solution was prepared as follows: 0.2 parts of bis(n-hexyldithiocarbamate)bismuth(III) was dissolved in 0.8 parts of tripropylene glycol and 1 part of 3,7-dimethyloctanol.
Embodiment 1
[0095] Example 1 : A master batch comprising 7774 parts of 6000 molecular weight ethylene oxide terminated poly(propylene oxide) triol, 1078 parts of 1,4-butanediol and 177 parts of silica-alumina was blended in a mechanical mixer Salt molecular sieve paste. 66.6 samples of this master batch were dispensed into plastic cups suitable for use on the FlakTex Speedmixer. 0.43 parts of the catalyst solution was added to the master batch and the mixture was mixed on the Speedmixer for 90 seconds. Then, 34.5 parts of modified MDI having an isocyanate functionality of about 2.1 were mixed into the polyol mixture for 60 seconds. The reaction mixture was then poured into a steel plate mold sprayed with an external mold release agent and preheated to 80°C. Measure tack free time and demold time. Tack-free time is the time after pouring that the composition no longer sticks to the metal spatula on which it is indicated. The demold time is the time necessary before the part can be dem...
Embodiment 2
[0096] Example 2 : The sample was prepared in the same manner as in Example 1, except that the amount of the catalyst solution was increased to 0.59 parts. The results are shown in Table 1.
[0097] Comparative sample A : Comparative Sample A was prepared in the same general manner as Example 1, except that the catalyst was 0.18 parts of titanium catalyst complex, which was supplied by Alfa Aesar, a Johnson Matthey company as Snapcure TM 2210 Commercial for sale.
[0098] Comparative sample B It was prepared as follows: 7 g of the polyol master batch were mixed with 50 microliters of a solution of 25.8 mmoles of bismuth tris(dodecylmercaptan) in 4 mL of toluene. This gave 0.323 mmol of catalyst. About 3.5 g of a 160.1 isocyanate equivalent uretonimine-modified diphenylmethane diisocyanate having an average of 2.1 isocyanate groups per molecule were added. This time is denoted as t=0. The resulting mixture was stirred at room temperature for 2 minutes and 45 seconds ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com