Oxidation treatment method for organic pollutants based on sulfur-oxygen radicals
A technology of organic pollutants and sulfur-oxygen free radicals, which is applied in the direction of oxidizing water/sewage treatment, water pollutants, water/sewage treatment, etc., can solve the problems of large ratio of oxidant and catalyst, high cost, etc., and achieve good oxidation effect and reaction Fast, highly oxidizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
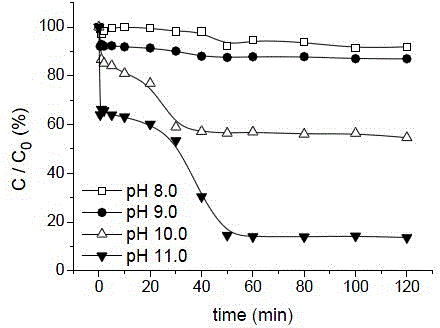
[0030] Prepare 400mL concentration of 8.6μM (2mg L -1 ) of bisphenol A solution, adding a concentration of 50mM MgSO 3 solution to form a mixed solution A, so that the MgSO in the mixed solution A 3 The concentration of the solution is 1 mM; quickly adjust the pH of the mixed solution A to about 11.4 with NaOH solution, add a magnet, and turn on the magnet stirrer. After that, quickly add Cu 2+ Solution (30mM, pH=2), forms mixed solution B, makes Cu in mixed solution B 2+The concentration is 0.1 mM, and the pH of the mixture B is 11.0 at this time. React under magnetic sub-stirring for 2 hours, the first hour before the reaction starts, take samples at intervals of 10 minutes, and one hour after the start of the reaction, take samples at intervals of 20 minutes, and the degradation efficiency of bisphenol A determined by liquid chromatography is 83%.
[0031] Similarly, the pH of the mixed liquid B was adjusted to 10.0, 9.0, and 8.0, and the degradation rates of bisphenol ...
Embodiment 2
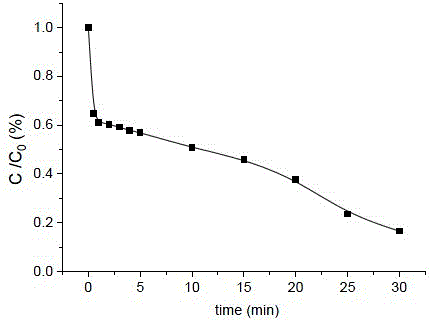
[0033] Degradation of orange Ⅱ is a micro reaction, and the concentration of 50mL is 28.6μM (10mg L -1 ) of Orange II solution, add NaHSO with a concentration of 50mM 3 solution to form a mixed solution A, so that NaHSO in mixed solution A 3 The concentration of the solution is 1mM; adjust the pH of the mixed solution A to about 11.4 with NaOH solution; then take 3mL of the mixed solution A in a cuvette, and quickly add Cu 2+ Solution (30mM, pH=2), forms mixed solution B, makes Cu in mixed solution B 2+ The concentration is 0.1 mM, and the pH of the mixture B is 11.0 at this time. Continuous measurement was carried out with a fiber optic spectrometer. After 30 minutes of reaction, the degradation rate of Orange II was 84%.
Embodiment 3
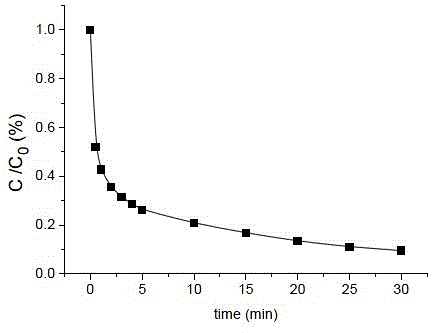
[0035] Degradation of acid black is a micro reaction, and the concentration of 50mL is 16.2μM (10mg L -1 ) acidic black solution, adding a concentration of 50mM ZnSO 3 solution to form a mixed solution A, so that ZnSO in the mixed solution A 3 The concentration of the solution is 1mM, and the pH of the mixed solution A is adjusted to about 11.4 with NaOH solution; then take 3mL of the mixed solution A in a cuvette, and quickly add Cu 2+ Solution (30mM, pH=2), forms mixed solution B, makes Cu in mixed solution B 2+ The concentration is 0.1 mM, and the pH of the mixture B is 11.0 at this time. Continuous measurement was carried out with a fiber optic spectrometer, and after 30 minutes of reaction, the degradation rate of the acid black was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com