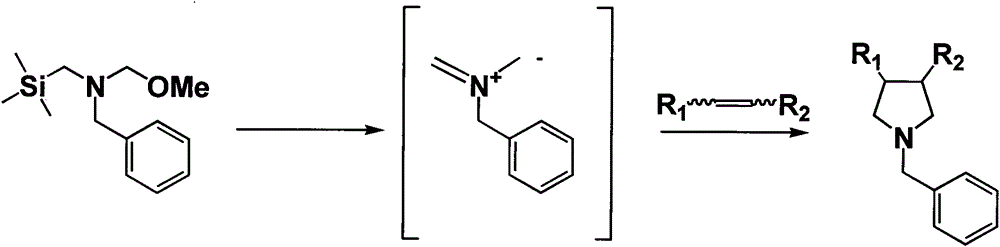
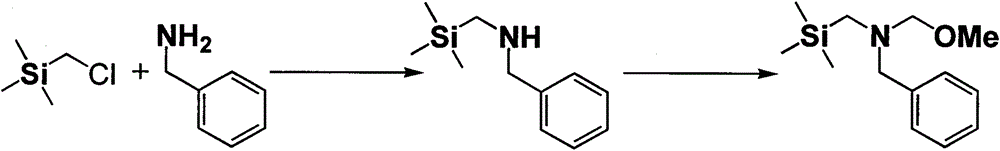
Synthesis method for N-methoxymethyl-N-(trimethylsilyl methyl)benzylamine
A technology of trimethylsilylmethylbenzylamine and trimethylchloromethylsilane, applied in the field of synthesis of N-methoxymethyl-N-benzylamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0014] Weighed trimethylchloromethylsilane (245.4 g, 2 mol) into a three-necked flask, evacuated it with nitrogen protection, and heated it up. When the temperature rises to 85 °C, start to add BnNH slowly 2 (556.4g, 5.2mol), the system was heated up slowly, the reaction temperature was controlled at 120°C, and the BnNH 2 After the dropwise addition, continue to maintain the system at 120°C for about 12 hours, and stop heating after the reaction is complete. After the system cools down to room temperature, remove the solids by filtration, soak and filter the filter cake with petroleum ether (150ml×2) until there is no product residue, collect the filter cake (benzylamine hydrochloride); the washings and filtrates are combined (about 1000ml) , washed three to four times with about 4L of water; the organic phase was mixed and dried with anhydrous potassium carbonate and anhydrous sodium sulfate, and the organic phase was distilled off under reduced pressure at 100°C-200°C, and ...
example 2
[0016] Weighed trimethylchloromethylsilane (245.4 g, 2 mol) into a three-necked flask, evacuated it with nitrogen protection, and heated it up. When the temperature rises to 85 °C, start to add BnNH slowly 2 (428g, 4mol), the system was heated up slowly, the reaction temperature was controlled at 100°C, and the BnNH 2 After the dropwise addition, continue to maintain the system at 200°C for about 5 hours, and stop heating after the reaction is complete. After the system cools down to room temperature, remove the solids by filtration, soak and filter the filter cake with petroleum ether (150ml×2) until there is no product residue, collect the filter cake (benzylamine hydrochloride); the washings and filtrates are combined (about 1000ml) , washed three to four times with about 4L of water; the organic phase was mixed and dried with anhydrous potassium carbonate and anhydrous sodium sulfate, and the organic phase was distilled off under reduced pressure at 100°C-200°C, and the c...
example 3
[0018] Weigh formaldehyde aqueous solution (37%) (198.7g, 2.52mol) in a 250ml three-necked flask, evacuate and ventilate nitrogen protection, cool to 0°C in an ice-salt bath, and then add trimethylsilylmethylbenzylamine (331.2g, 1.72mol) was added dropwise into a three-necked flask, the temperature was controlled at 0°C, and the reaction was stirred for 1 h after the injection was completed, then anhydrous potassium carbonate (187.4g, 1.39mol) was added in batches at -5°C, and methanol (229.2 g, 7.15mol), stop cooling, warm up to room temperature, and stir overnight. After the reaction was completed, the filtrate was allowed to stand and separated into layers, and the upper layer was distilled off under reduced pressure to remove a small amount of methanol, and concentrated to obtain about 364.3 g of crude product N-methoxymethyl-N-(trimethylsilylmethyl)benzylamine. Under reduced pressure distillation, 77-80 degree fractions were collected under 0.5 mm Hg pressure to obtain 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com