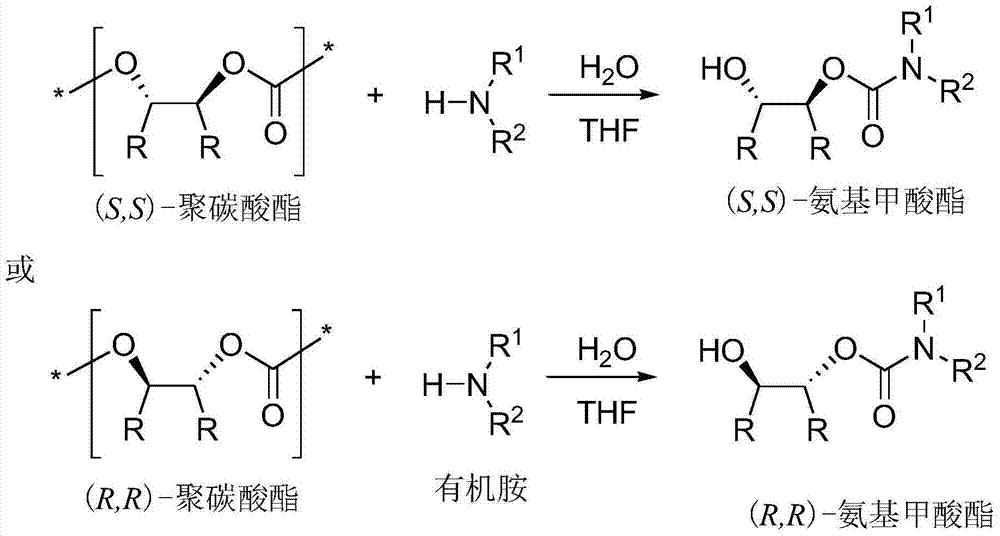
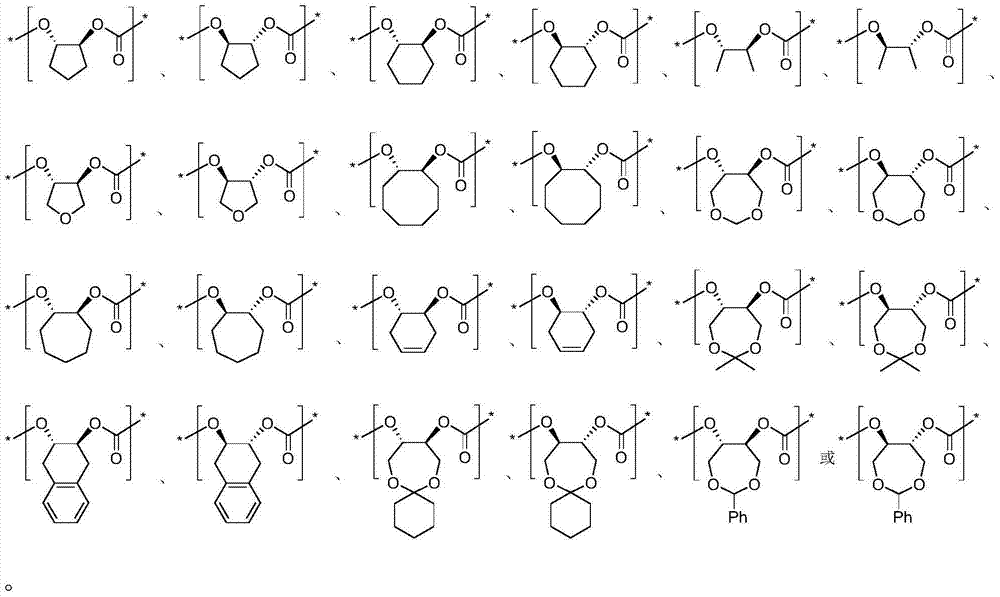
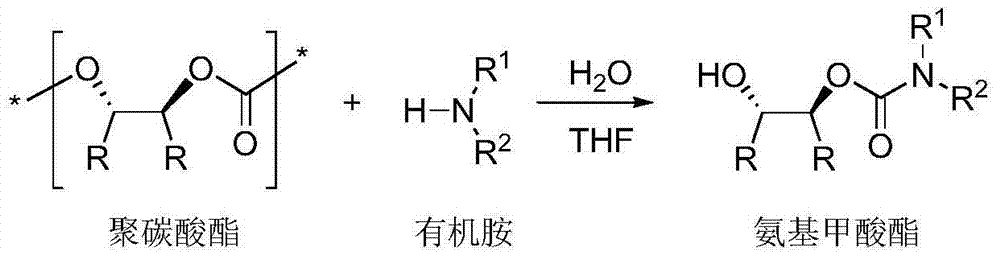
Synthesis method for chiral carbamic acid ester and derivative thereof
A technology of carbamate and synthesis method, which is applied in the field of synthesis of chiral carbamate and its derivatives, can solve the problems of harsh reaction conditions, low reaction yield, single structure of carbamate derivatives, etc. Achieve the effect of mild reaction conditions, high separation yield, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The specific implementation manner of the present invention will be described in detail below in conjunction with the technical solutions.
[0024] In a 50 mL round bottom flask, polycarbonate, organic amine and water were dissolved in THF solution. Stir at the agreed temperature for the appointed time, stop the reaction, wait for the reaction solution to cool to room temperature, add 1M dilute hydrochloric acid, extract the organic phase three times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, spin Dry solvent, apply column chromatography (silica gel column; developer: petroleum ether / ethyl acetate = 1 / 1-10 / 1) to separate and purify, and spin off the solvent to obtain trans-2-hydroxycarbamate and its derivatives things.
[0025] Table 1 Ammonolysis reaction of high stereoregularity polycarbonate
[0026]
[0027]
[0028]
[0029]
[0030]
[0031] Note 1: The molar ratio of organic amine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com