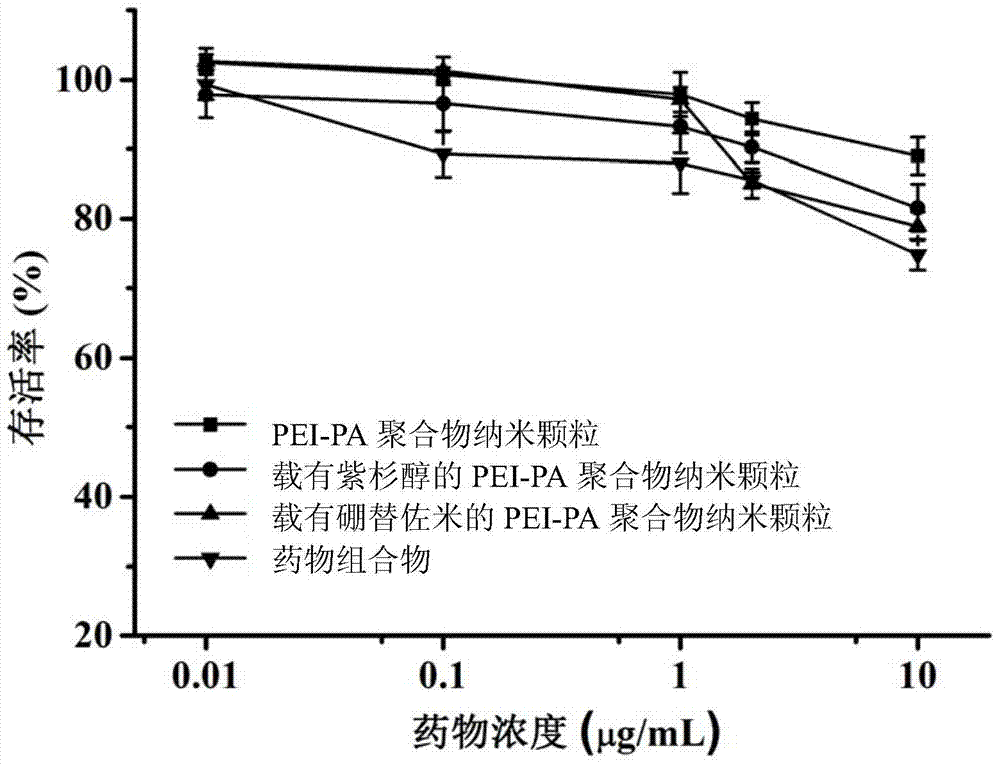
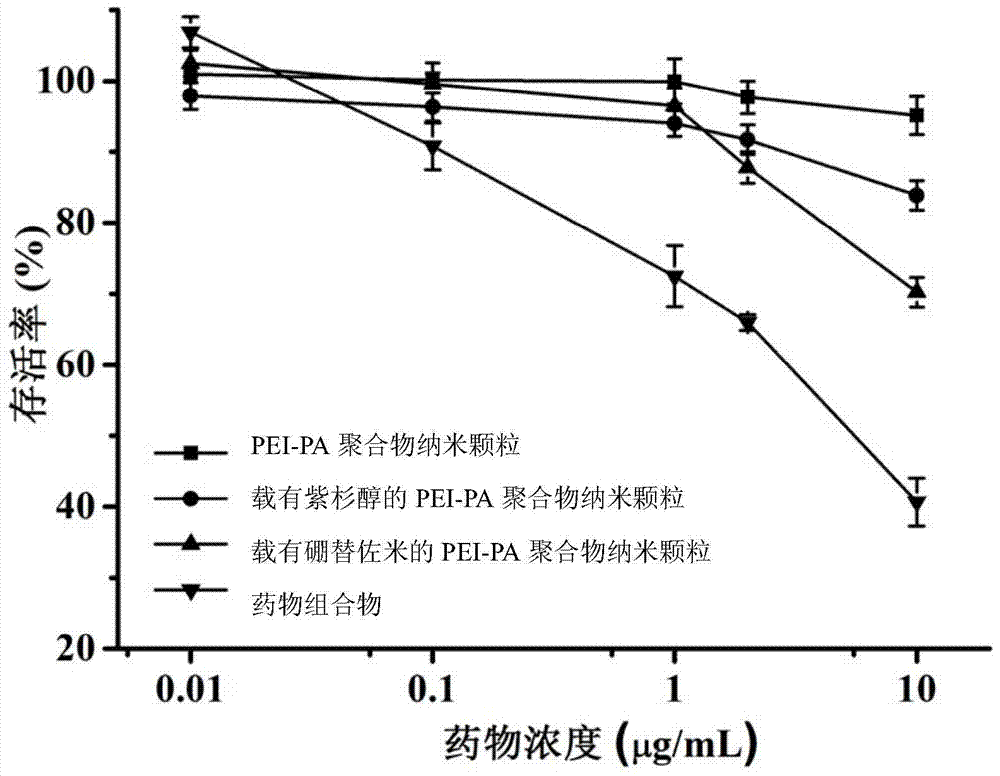
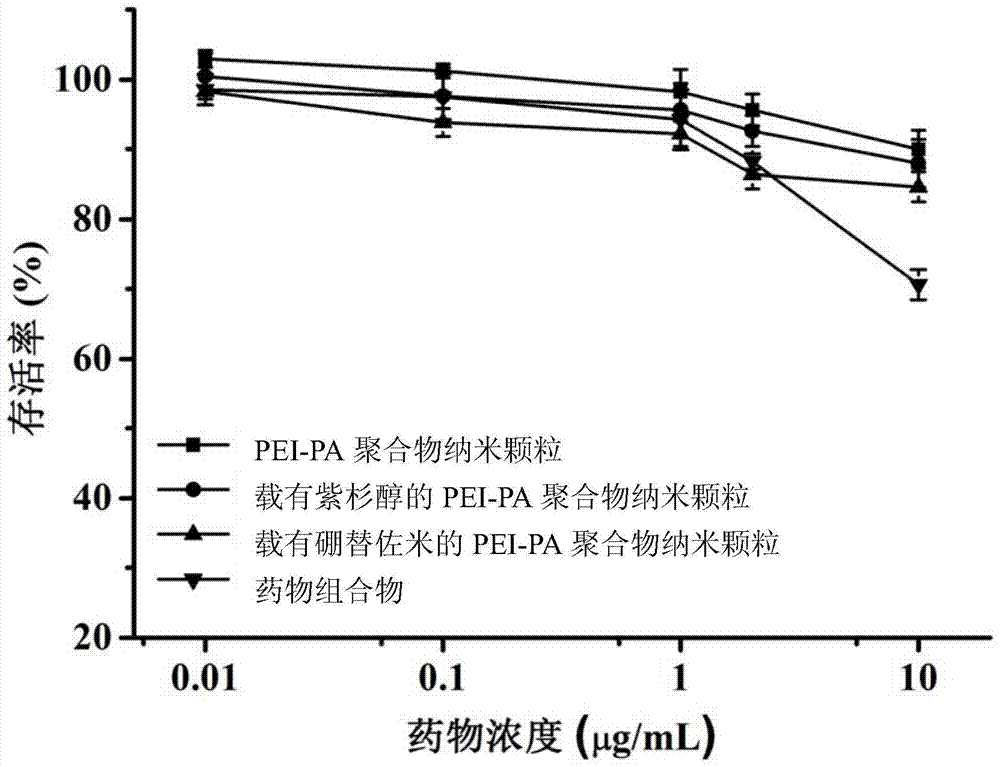
A kind of pharmaceutical composition and its preparation method and application
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, boron compound active ingredients, etc., can solve the problems of poor stability, low bioavailability, high normal tissue toxicity, etc., and achieve narrow distribution and high drug loading capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0040] Example 1-1: Synthesis of PEI-PA
[0041]Synthesis of PEI-PA: Weigh 256 mg of dry palmitic acid, pour it into a three-necked flask, add 15 mL of dimethylformamide (DMF), completely dissolve the palmitic acid, and then weigh 380 mg of HATU (2-(7- Nitrobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) was added to the DMF solution of palmitic acid, and 129μL DIEA(N,N- diisopropylethylamine), reacted overnight under magnetic stirring. Weighed 250mg PEI and dissolved it in 15mL DMF. After complete dissolution, the activated palmitic acid mixed solution was added dropwise to the PEI solution, and reacted for 12 hours to obtain the PEI-PA polymer.
Embodiment 1-2
[0042] Example 1-2: Synthesis of PEI-PA
[0043] Synthesis of PEI-PA: Weigh 256 mg of dry palmitic acid, pour it into a three-necked flask, add 15 mL of dimethylformamide (DMF), completely dissolve the palmitic acid, then weigh 570 mg of HATU (2-(7- Nitrobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) was added to the DMF solution of palmitic acid, and 104μL DIEA(N,N- Diisopropylethylamine), reacted under magnetic stirring for 6 hours. Weighed 500mg PEI and dissolved it in 15mL DMF. After complete dissolution, the activated palmitic acid mixed solution was added dropwise to the PEI solution and reacted for 24 hours to obtain the PEI-PA polymer.
Embodiment 1-3
[0044] Example 1-3: Synthesis of PEI-PA
[0045] Synthesis of PEI-PA: Weigh 256 mg of dry palmitic acid, pour it into a three-necked flask, add 15 mL of dimethylformamide (DMF), completely dissolve the palmitic acid, then weigh 304 mg of HATU (2-(7- Nitrobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) was added to the DMF solution of palmitic acid, and 194μL DIEA(N,N- Diisopropylethylamine), reacted for 20 hours under magnetic stirring. 130mg PEI was weighed and dissolved in 15mL DMF. After complete dissolution, the activated palmitic acid mixed solution was added dropwise to the PEI solution and reacted for 8 hours to obtain the PEI-PA polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com