Water-soluble dual-targeting near-infrared fluorescent probe and preparation method thereof
A fluorescent probe and near-infrared technology, applied in the field of near-infrared fluorescent probes, can solve the problems of inability to achieve water solubility, insufficient targeting of intracellular substructures, etc., to ensure biocompatibility and use effects, and simple synthesis steps. , the effect of a simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A method for preparing a water-soluble dual-target near-infrared fluorescent probe, the steps are as follows:
[0045] 1) Synthesis of 1,4-dibromo-2,5-dicarbenyl
[0046] To a suspension of 11.4mmol 1,4-dibromo-2,5-xylene, 15mL acetic acid and 30mL acetic anhydride was slowly added dropwise 11mL concentrated sulfuric acid and 45mmol CrO 3 Granules, the resulting mixture was stirred at 0°C for 8 h, poured into ice water and filtered, the obtained white solid was hydrolyzed with a mixture of 15 mL of water, 15 mL of ethanol and 1.5 mL of sulfuric acid under reflux overnight, and then recrystallized by dichloromethane / n-hexane , to obtain the light yellow solid product 1,4-dibromo-2,5-dicarbenyl, and the synthetic route is shown in the following formula.
[0047]
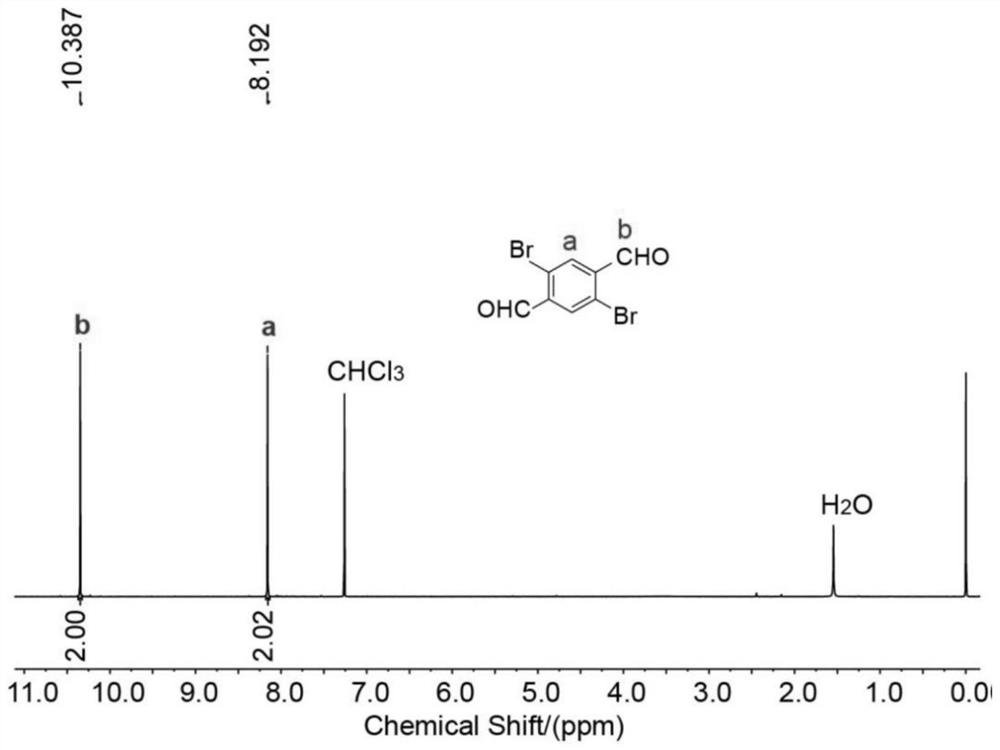
[0048] The 1,4-dibromo-2,5-dicarboxylate prepared in Example 1, that is, the compound 1 1 The H NMR nuclear magnetic pattern is as follows figure 1 as shown, 1 H NMR (400MHz, CDCl 3 ,295K) δ10.39(s,2H),8...
Embodiment 2
[0064] A method for preparing a water-soluble dual-target near-infrared fluorescent probe, the steps are as follows:
[0065] 1) Synthesis of 1,4-dibromo-2,5-dicarbenyl (1)
[0066] To a suspension of 11.8mmol 1,4-dibromo-2,5-xylene, 15.5mL acetic acid and 31mL acetic anhydride was slowly added dropwise 11.4mL concentrated sulfuric acid and 46.5mmol CrO 3 particles. The resulting mixture was stirred at 0 °C for 9 h, poured into ice water and filtered. A mixture of the obtained white solid and 15.5 mL of water, 15.5 mL of ethanol and 1.55 mL of sulfuric acid was hydrolyzed under reflux overnight, and then recrystallized by dichloromethane / n-hexane to obtain a pale yellow solid product 1,4-dibromo-2, 5-two Tsuen base.
[0067]
[0068] 2) Synthesis of compound 2
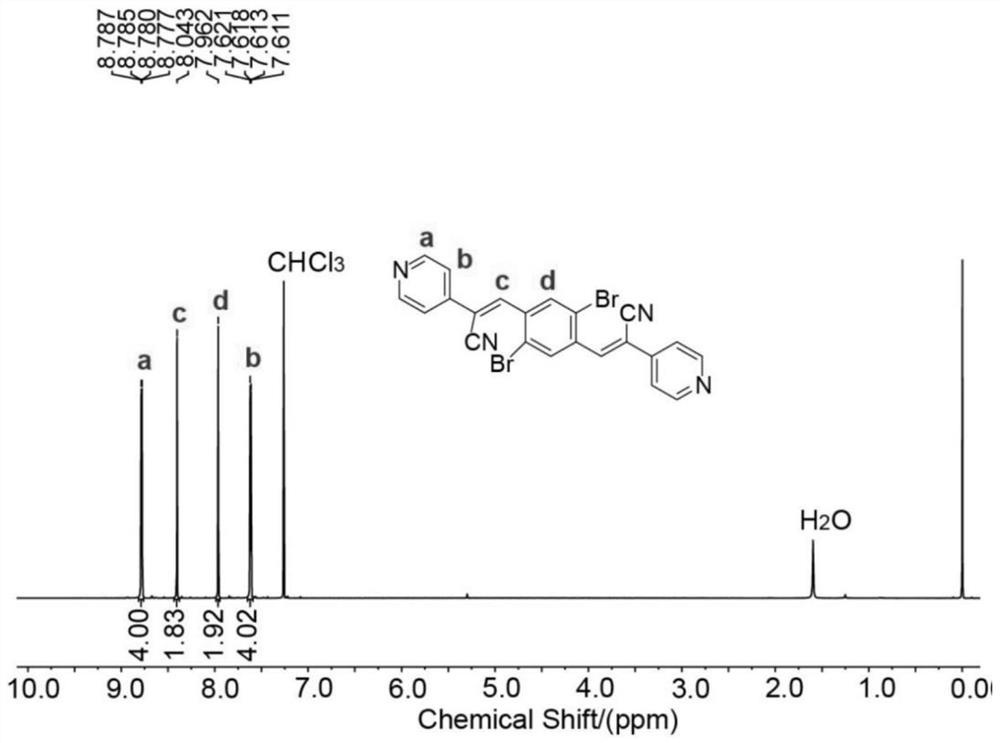
[0069] 3.1mmol 1,4-dibromo-2,5-dicarboxylate and 6.8mmol 2-(pyridin-4-yl)acetonitrile hydrochloride were dissolved in a mixed solution of 20.5mL ethanol and 20.5mL dichloromethane, then 13.5 mmol of triethylami...
Embodiment 3
[0078] A method for preparing a water-soluble dual-target near-infrared fluorescent probe, the steps are as follows:
[0079] 1) Synthesis of 1,4-dibromo-2,5-dicarbenyl (1)
[0080] To a suspension of 11.6mmol 1,4-dibromo-2,5-xylene, 15.2mL acetic acid and 30.5mL acetic anhydride was slowly added dropwise 11.2mL concentrated sulfuric acid and 46mmol CrO 3 (4.5~4.65g, 45~46.5mmol) granules. The resulting mixture was stirred at 0 °C for 8.5 h, poured into ice water and filtered. A mixture of the obtained white solid and 15.2 mL of water, 15.2 mL of ethanol and 1.52 mL of sulfuric acid was hydrolyzed under reflux overnight, and then recrystallized by dichloromethane / n-hexane to obtain a pale yellow solid product 1,4-dibromo-2, 5-two Tsuen base.
[0081]
[0082] 2) Synthesis of Compound 2
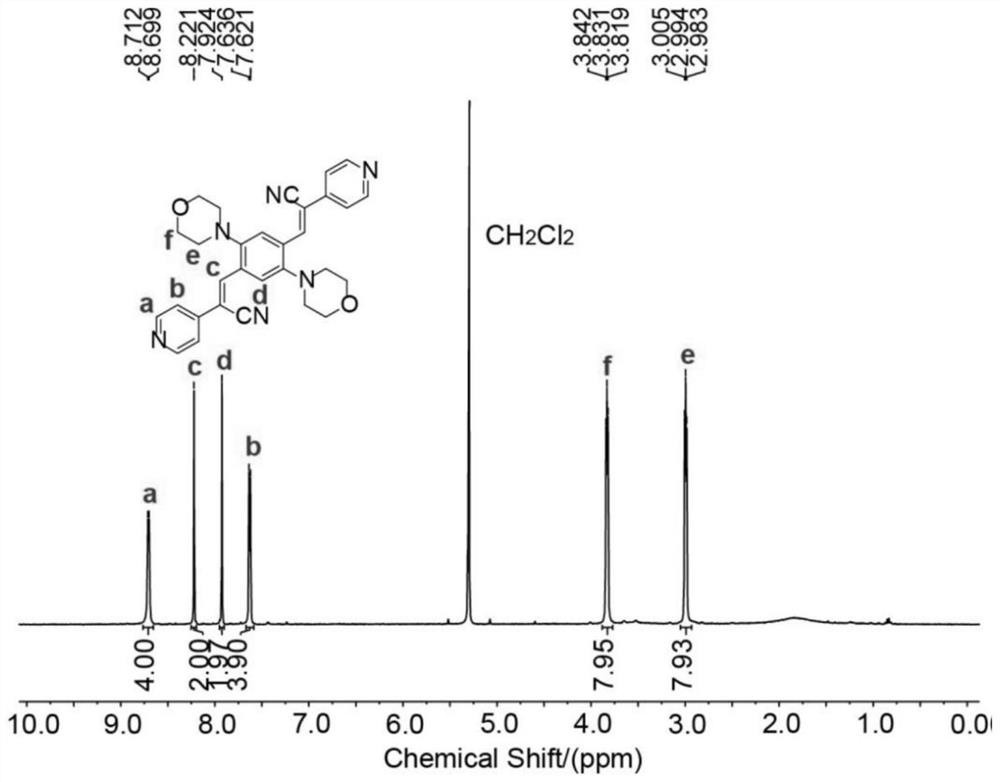
[0083] Dissolve 3.15mmol 1,4-dibromo-2,5-dicarboxylate, 6.93mmol 2-(pyridin-4-yl)acetonitrile hydrochloride in a mixed solution of 21mL ethanol and 21mL dichloromethane, and then add 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com