Antibacterial composite hydrogel as well as preparation method and application thereof
A composite hydrogel and water-soluble gel technology, which is applied in capsule delivery, pharmaceutical formulation, medical science, etc., can solve bacterial drug resistance and other problems, and achieve antibacterial performance, biocompatibility, and good biocompatibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of methacrylamide gelatin (GelMA), the steps are as follows:
[0051] Weigh 20g of gelatin (Gel) and dissolve it in 250mL of distilled water. After dissolving at 60°C, add 12mL of methacrylic anhydride, react at 60°C for 8 hours, and dialyze with distilled water for 3 to 5 days (molecular weight cut-off: 3500D). , put the solution in a 60°C water bath, add 2g of activated carbon for decolorization treatment for 15min, centrifuge at 8000rpm for 5min, filter with neutral filter paper, and freeze-dry at -80°C to obtain GelMA.
Embodiment 2
[0053] The preparation of silver nanoparticles (Ag NPs), the steps are as follows:
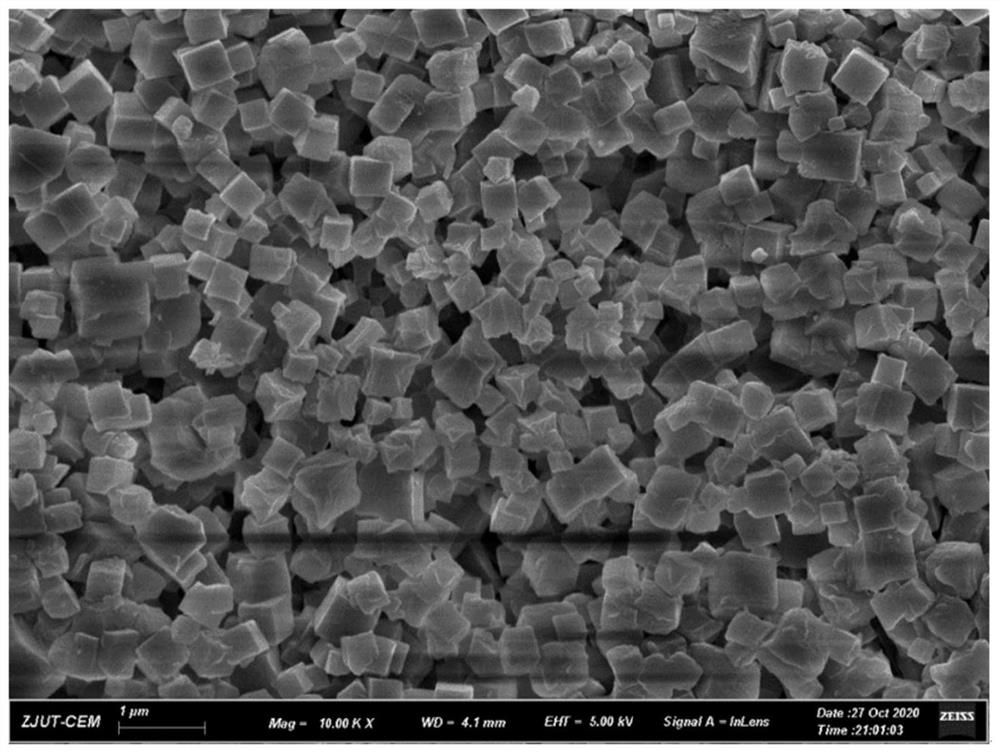
[0054] (1) Synthesis of CD-MOF: γ-cyclodextrin (γ-CD, 97.3g) and potassium hydroxide (KOH, 33.6g) were dissolved in 3L of purified water at a molar ratio of 1:8. After the solution was filtered through a 0.45 μm membrane filter, it was added to the reaction vessel. Methanol (1.8 L) was heated by a distillation apparatus, and its vapor was diffused in an aqueous solution at 50° C. for 20 min. Then polyethylene glycol (Mo=20000, 38.4 g) was added and stirred for 10 min. The solution was incubated overnight at 15°C to trigger crystallization. The precipitate was washed with ethanol several times, and dried in a vacuum oven at 40 °C overnight to obtain CD-MOF crystals.
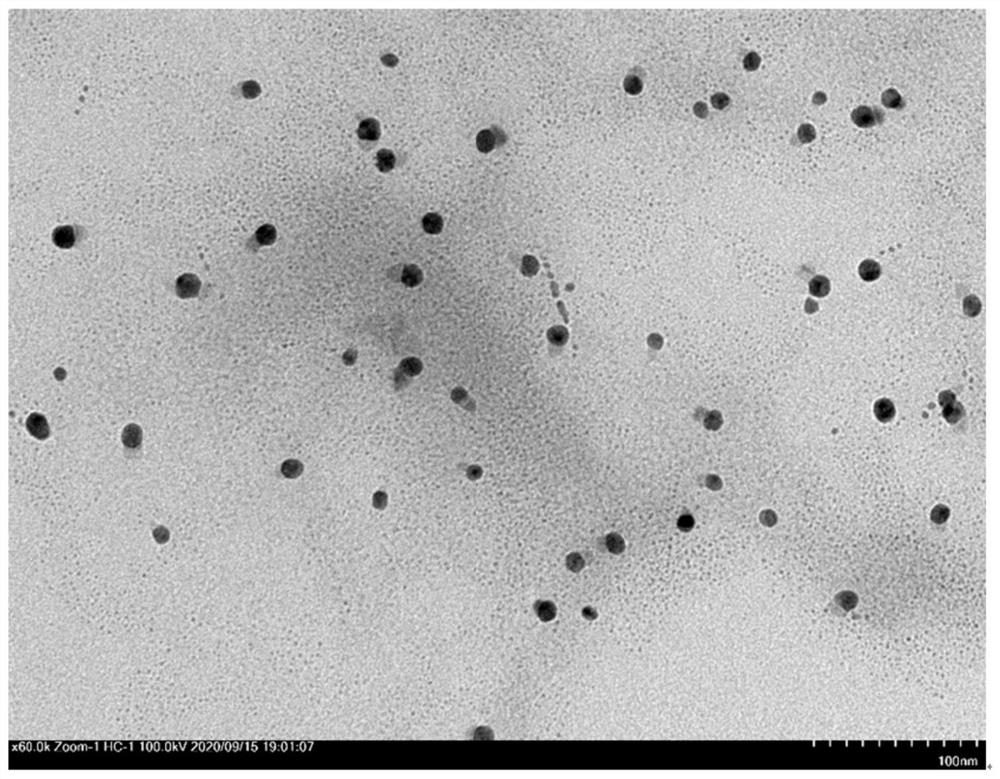
[0055] (2) Synthesis of Ag NPs: Ag NPs were synthesized by solvent impregnation and modified reaction-diffusion method. Specifically, CD-MOF crystals (600 mg) were suspended in 1.5 mL of acetonitrile, and then soaked in AgNO ...
Embodiment 3
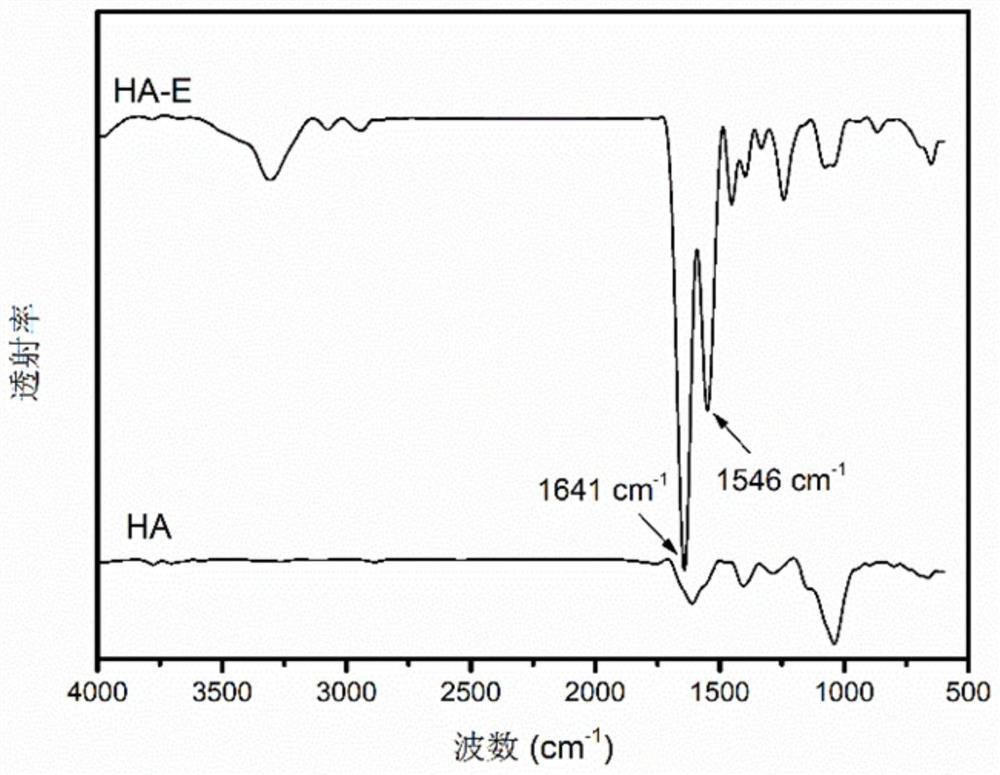
[0057] The preparation of modified hyaluronic acid (HA-E), the steps are as follows:
[0058] Dissolve 145 μL of aminoacetaldehyde diethyl acetal in 1.2 mL of a mixture of methanesulfonic acid (MSA) and tetrahydrofuran (THF) (MSA:THF=1:5, v / v) at 4°C to obtain aminoacetaldehyde diethyl acetal mixture. EGCG (2.29 g) was dissolved in 3.8 mL THF and 1.7 μL MSA, and then the aminoacetaldehyde diethyl acetal mixture was added under stirring, and stirred overnight at room temperature in the dark. The resulting mixture was concentrated by rotary pump for 20 minutes and dried under vacuum at room temperature overnight. The product was dissolved in 10 mL of distilled water, extracted and purified 5 times with 10 mL of ethyl acetate, the organic phase was dehydrated with a sufficient amount of anhydrous sodium sulfate, and after filtration, the organic solvent was removed by a rotary evaporator to obtain a dimer of EGCG. Next, weigh 5 g of hyaluronic acid (HA), dissolve it in 100 mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com