A kind of water-soluble dual-targeting near-infrared fluorescent probe and preparation method thereof
A fluorescent probe and near-infrared technology, applied in the field of near-infrared fluorescent probes, can solve the problems of insufficient substructure in targeted cells and inability to achieve water solubility, achieve simple synthesis steps, ensure biocompatibility and use effects , the effect of a simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A preparation method of a water-soluble dual-targeting near-infrared fluorescent probe, the steps are as follows:
[0045] 1) Synthesis of 1,4-dibromo-2,5-dialdehyde benzene
[0046] To a suspension of 11.4 mmol 1,4-dibromo-2,5-xylene, 15 mL acetic acid and 30 mL acetic anhydride was slowly added dropwise 11 mL concentrated sulfuric acid and 45 mmol CrO 3 The resulting mixture was stirred at 0 °C for 8 h, poured into ice water and filtered, and the resulting white solid was hydrolyzed overnight with a mixture of 15 mL of water, 15 mL of ethanol and 1.5 mL of sulfuric acid, and then recrystallized from dichloromethane / n-hexane. , to obtain a light yellow solid product, 1,4-dibromo-2,5-dialdehyde benzene, and the synthetic route is shown in the following formula.
[0047]
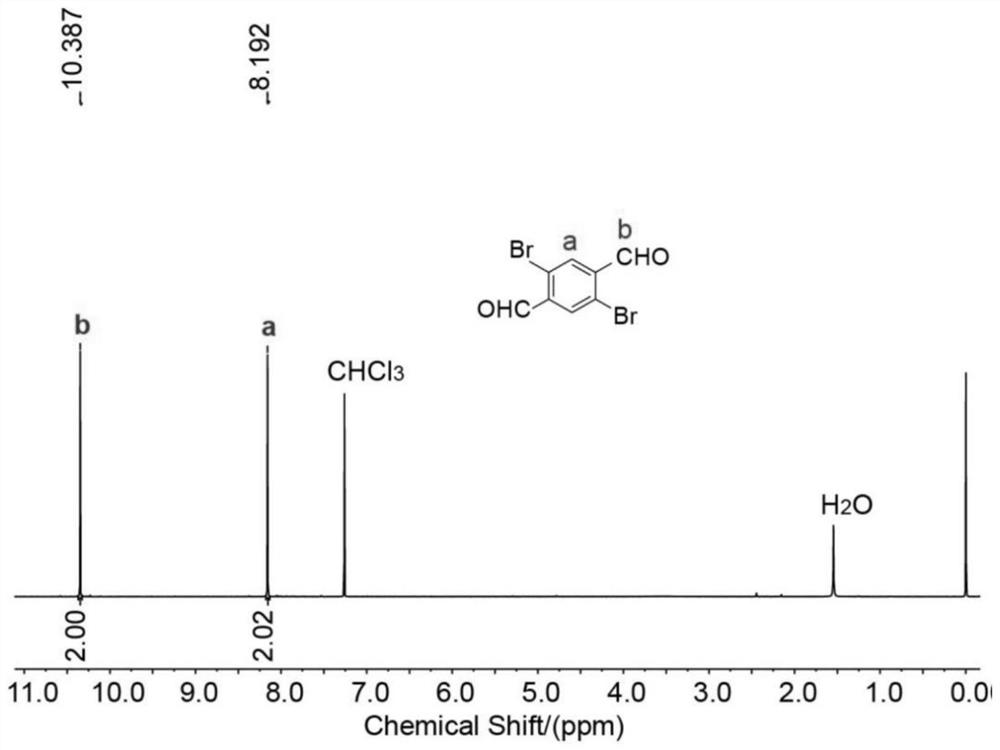
[0048] The 1,4-dibromo-2,5-dialdehyde benzene prepared in Example 1 is the compound 1 1 H NMR nuclear magnetic map such as figure 1 shown, 1 H NMR (400MHz, CDCl 3 ,295K)δ10.39(s,2H),8.19(s,2H)....
Embodiment 2
[0064] A preparation method of a water-soluble dual-targeting near-infrared fluorescent probe, the steps are as follows:
[0065] 1) Synthesis of 1,4-dibromo-2,5-dialdehyde benzene (1)
[0066] To a suspension of 11.8 mmol 1,4-dibromo-2,5-xylene, 15.5 mL acetic acid and 31 mL acetic anhydride was slowly added dropwise 11.4 mL concentrated sulfuric acid and 46.5 mmol CrO 3 particles. The resulting mixture was stirred at 0 °C for 9 h, poured into ice water and filtered. The obtained white solid was hydrolyzed by refluxing overnight with a mixture of 15.5 mL of water, 15.5 mL of ethanol and 1.55 mL of sulfuric acid, and then recrystallized from dichloromethane / n-hexane to obtain a pale yellow solid product 1,4-dibromo-2, 5-Dialdehyde benzene.
[0067]
[0068] 2) Synthesis of compound 2
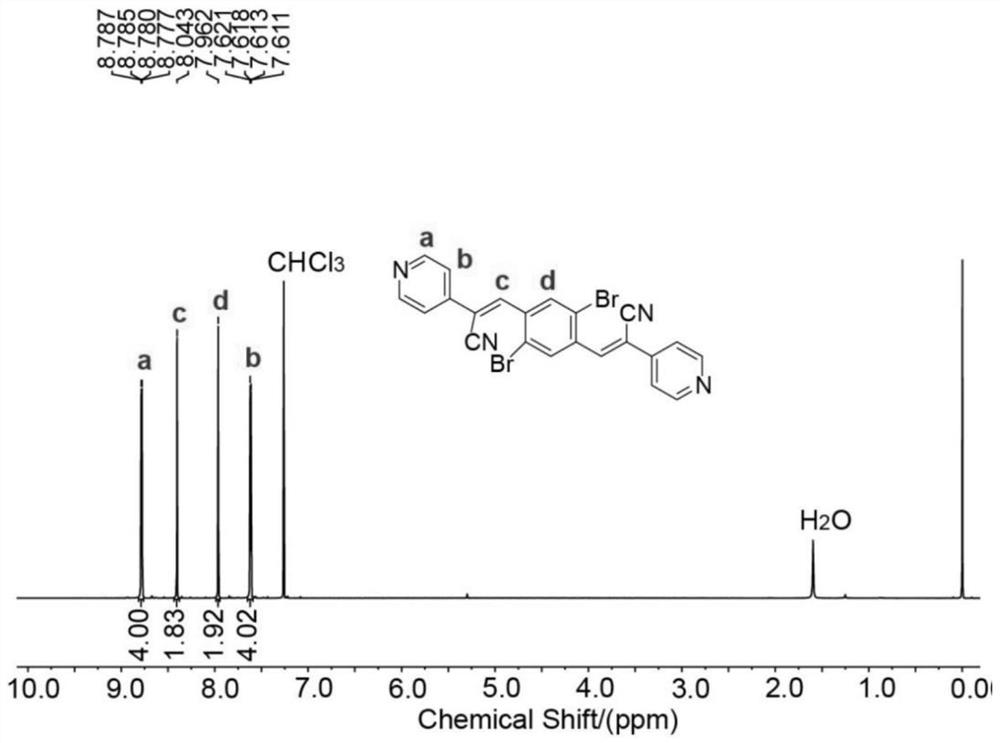
[0069] 3.1 mmol of 1,4-dibromo-2,5-dialdehyde benzene and 6.8 mmol of 2-(pyridin-4-yl)acetonitrile hydrochloride were dissolved in a mixed solution of 20.5 mL of ethanol and 20.5 mL of di...
Embodiment 3
[0078] A preparation method of a water-soluble dual-targeting near-infrared fluorescent probe, the steps are as follows:
[0079] 1) Synthesis of 1,4-dibromo-2,5-dialdehyde benzene (1)
[0080] To a suspension of 11.6 mmol 1,4-dibromo-2,5-xylene, 15.2 mL acetic acid and 30.5 mL acetic anhydride was slowly added dropwise 11.2 mL concentrated sulfuric acid and 46 mmol CrO 3 (4.5-4.65 g, 45-46.5 mmol) granules. The resulting mixture was stirred at 0°C for 8.5 h, poured into ice water and filtered. The obtained white solid was hydrolyzed by refluxing overnight with a mixture of 15.2 mL of water, 15.2 mL of ethanol and 1.52 mL of sulfuric acid, and then recrystallized from dichloromethane / n-hexane to obtain a pale yellow solid product 1,4-dibromo-2, 5-Dialdehyde benzene.
[0081]
[0082] 2) Synthesis of compound 2
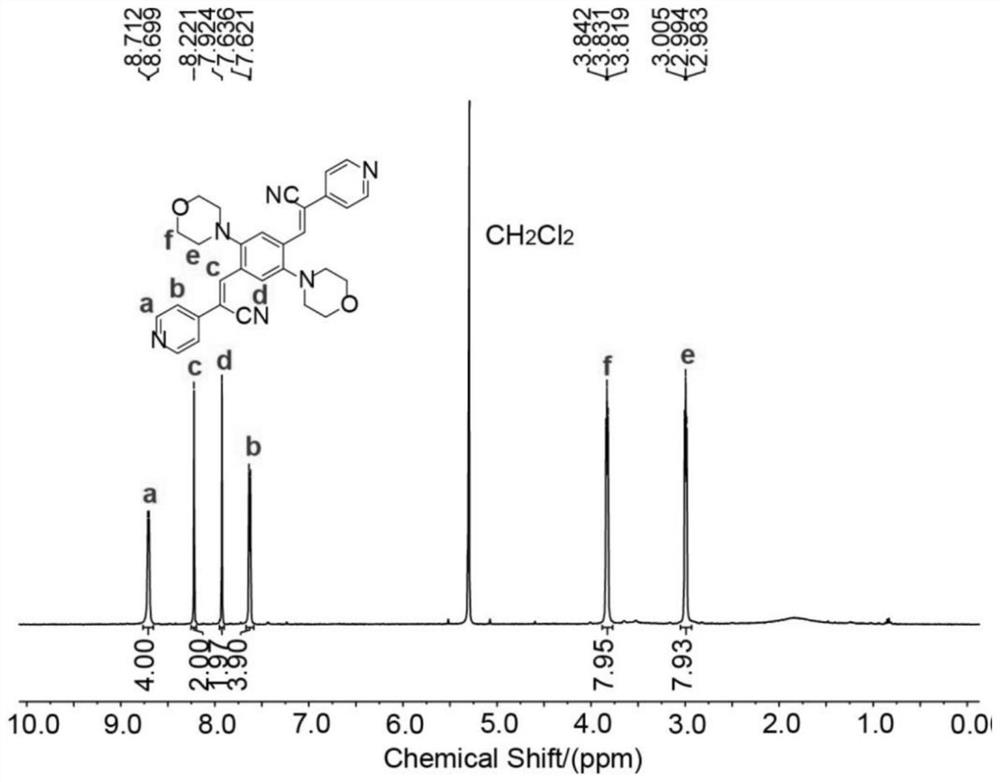
[0083] Dissolve 3.15 mmol of 1,4-dibromo-2,5-dialdehyde benzene and 6.93 mmol of 2-(pyridin-4-yl)acetonitrile hydrochloride in a mixed solution of 21 mL of etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com