4-hydroxyl bishydroxycoumarin compound and application thereof
A dicoumarin and compound technology, applied in 3 fields, can solve the problems of toxicity, coumarin compound toxicity, low success rate of antibacterial effect, etc., and achieve strong bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
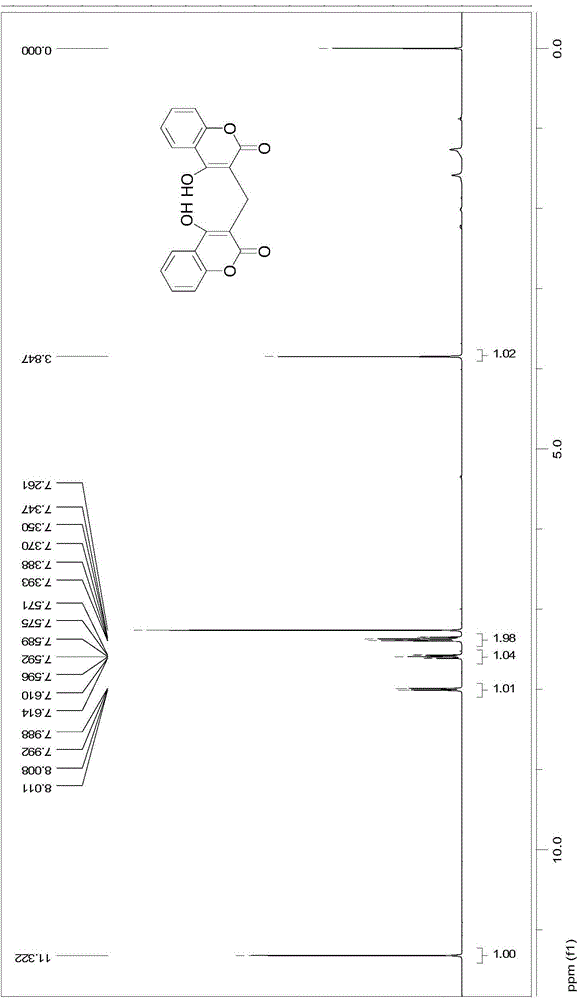
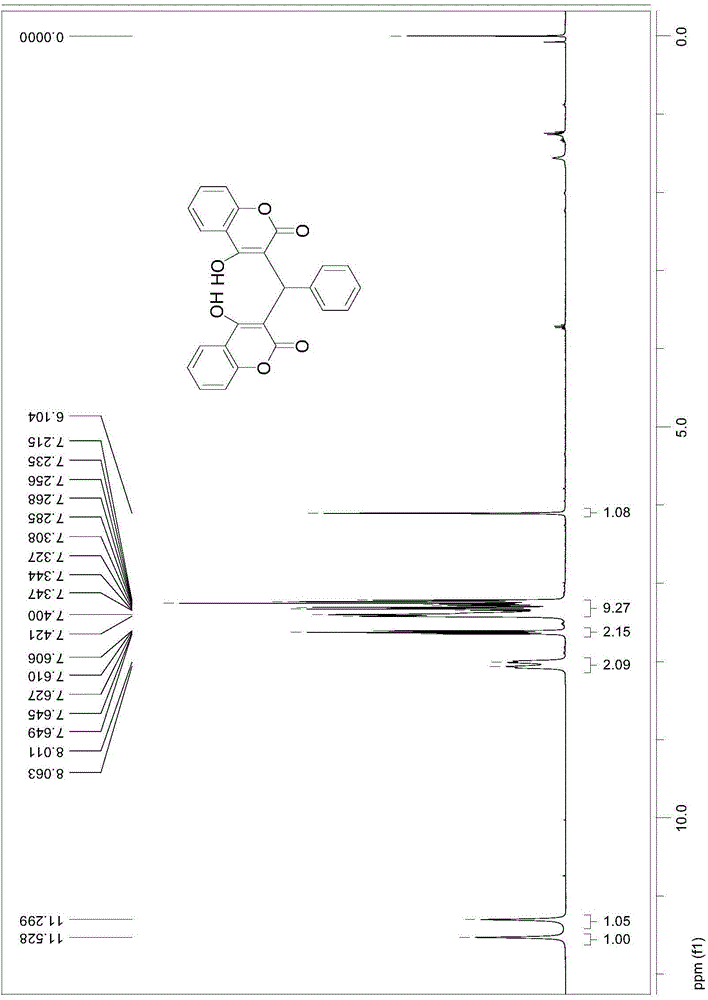
[0035] This embodiment synthetic general formula is as the compound shown in formula II:
[0036]
[0037] in:
[0038] (1) Only benzene ring, that is, no substituent R;
[0039] (2) R=3-F, 4-F, 3,4-2F, 3,5-2F, 3-I, 4-I, 3-CF 3 , 4-CF 3 , 3, 5-2CF 3 , 3-CH 3 , 4-CH 3 , 3, 4-2CH 3 , 3-OCH 3 , 4-OCH 3 , 3,4-OCH 3 or 3,5-2OCH 3
[0040] The synthesis method is:
[0041] A: Add 10g of 4-hydroxycoumarin and 100mL of absolute ethanol into a 250mL three-necked flask, and heat until the 4-hydroxycoumarin is dissolved.
[0042] B: Add a series of aromatic aldehydes with different substituents, heat and reflux for 3-4 hours.
[0043] C: Solid particles are precipitated. Continue heating for about 1 hour. After the reaction is completed, filter with suction after natural cooling, and then recrystallize with 95% ethanol to finally obtain pure white granular crystals.
[0044] Structure Identification:
[0045] Using organic spectra such as mass spectrometry (MS), nuclear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com