Immobilized enzyme carrier as well as preparation method and application thereof
An immobilized enzyme carrier and immobilized enzyme technology, which can be applied in the direction of immobilization on or in an inorganic carrier, can solve the problems of reducing the apparent enzyme activity, the limited types of functional groups on the surface of magnetic nanoparticles, and the deformation of the enzyme molecular structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method for an immobilized enzyme carrier, comprising the following steps:
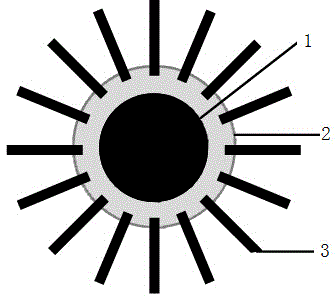
[0033] (1) Preparation of magnetic nanoparticles: Preparation of magnetic Fe by chemical co-precipitation method 3 o 4 Nanoparticles, the average particle size is controlled to be 50nm.
[0034] (2) Preparation of a hydrophilic sol-gel compound rich in carbon nanotubes: 1.0 g of agarose was dissolved in 45 mL of water, heated to boiling to form a sol. 0.5 g of carbon nanotubes (50 nm in length) were added to the boiling agarose solution, and ultrasonically dispersed at 220w at 80° C. for 30 minutes to obtain a hydrophilic sol-gel compound solution rich in carbon nanotubes.
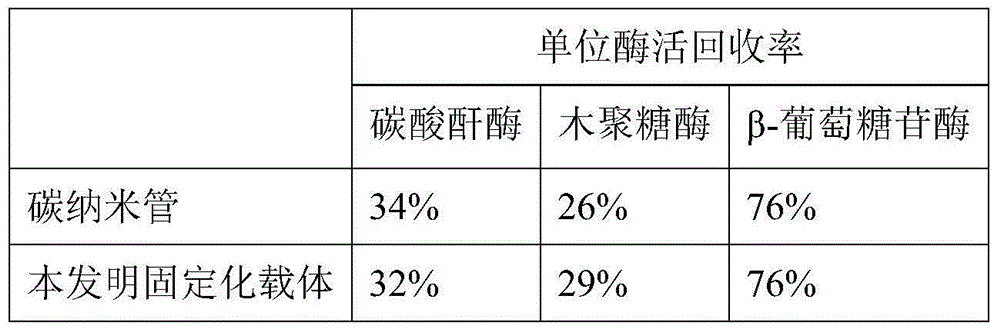
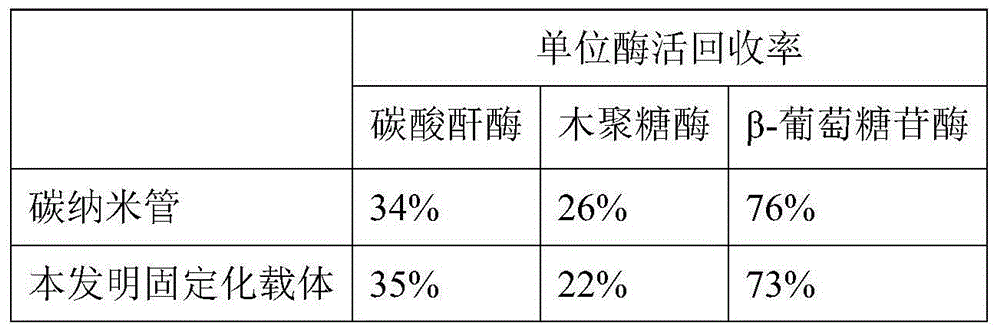
[0035] (3) Carry out graft modification of carbon nanotubes to the magnetic nanoparticles prepared in step (1): add 0.5g of magnetic nanoparticles to 45mL of hydrophilic sol-gel compound solution rich in carbon nanotubes, 80 Ultrasonic dispersion at ℃ 220w for 30min to obtain magnetic Fe 3 o 4 , carbon nan...
Embodiment 2
[0049] A preparation method for an immobilized enzyme carrier, comprising the following steps:
[0050] (1) Preparation of magnetic nanoparticles: Preparation of magnetic Fe by chemical co-precipitation method 3 o 4 Nanoparticles, the average particle size is controlled to be 200nm.
[0051] (2) Preparation of a hydrophilic sol-gel compound rich in carbon nanotubes: 1.0 g of agarose was dissolved in 45 mL of water, heated to boiling to form a sol. 4.5 g of carbon nanotubes (250 nm in length) were added to the boiling agarose solution, and ultrasonically dispersed at 220w at 80° C. for 30 minutes to obtain a hydrophilic sol-gel compound solution rich in carbon nanotubes.
[0052] (3) Carry out graft modification of carbon nanotubes to the magnetic nanoparticles prepared in step (1): add 2.25g of magnetic nanoparticles to 45mL of the hydrophilic sol-gel compound solution rich in carbon nanotubes, 80 Ultrasonic dispersion at ℃ 220w for 30min to obtain magnetic Fe 3 o 4 , car...
Embodiment 3
[0066] A preparation method for an immobilized enzyme carrier, comprising the following steps:
[0067] (1) Preparation of magnetic nanoparticles: Preparation of magnetic Fe by chemical co-precipitation method 3 o 4 Nanoparticles, the average particle size is controlled to be 100nm.
[0068] (2) Preparation of a hydrophilic sol-gel compound rich in carbon nanotubes: 1.0 g of chitosan was dissolved in 45 mL of water, heated to boiling to form a sol. 0.45 g of carbon nanotubes (500 nm in length) were added to the boiling agarose solution, and ultrasonically dispersed at 220w at 80° C. for 30 min to obtain a hydrophilic sol-gel compound solution rich in carbon nanotubes.
[0069] (3) Carry out graft modification of carbon nanotubes to the magnetic nanoparticles prepared in step (1): add 0.45g of magnetic nanoparticles to 45mL of the hydrophilic sol-gel compound solution rich in carbon nanotubes, 80 Ultrasonic dispersion at ℃ 220w for 30min to obtain magnetic Fe 3 o 4 , carbon ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com