Medicine composition for treating skin diseases as well as preparation method and application thereof
A composition and skin disease technology, applied in the field of pharmaceutical compositions for the treatment of skin diseases, can solve the problems of cumbersome and complicated compound ingredients, unfavorable large-scale preparation, etc., and achieve the goals of wide application range, quick treatment effect, and reduced drug cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The tablet preparation of embodiment 1 pharmaceutical composition of the present invention
[0032] The raw materials used are:
[0033] Doramectin 1g
[0034] Terbinafine 1g
[0035] Microcrystalline Cellulose 98g
[0036] A total of 200 tablets
[0037] Preparation method: Mix 1g of doramectin, 1g of terbinafine and 98g of microcrystalline cellulose evenly, and then directly compress into tablets by dry method, making a total of 200 tablets, each weighing 0.5g.
Embodiment 2
[0038] The spray preparation of embodiment 2 pharmaceutical compositions of the present invention
[0039] The raw materials used are:
[0040] Doramectin 1g
[0041] Terbinafine 1.5g
[0042] Ethylparaben 0.2g
[0043] Butylparaben 0.1g
[0044] Propylene glycol 80g
[0045] Add ethanol to 100g
[0046] Preparation method: Dissolve 1g doramectin and 0.5g terbinafine in 80g propylene glycol, add 0.2g ethylparaben and 0.1g butylparaben, add ethanol to prepare 100g solution, and pack in Available in a spray bottle.
Embodiment 3
[0047] The ointment preparation of embodiment 3 pharmaceutical composition of the present invention
[0048] The raw materials used are:
[0049] Doramectin 1g
[0050] Terbinafine 2g
[0051] Appropriate amount of vegetable oil
[0052] Beeswax 20g
[0053] Lanolin 17g
[0054] Ethylparaben 0.2g
[0055] Butylparaben 0.1g
[0056] Total 100g
[0057] Preparation method: Dissolve 1g doramectin and 2g terbinafine in 50g hot vegetable oil, then add 20g beeswax, 17g lanolin in sequence, finally add 0.2g ethylparaben and 0.1g butylparaben, add The remaining vegetable oil is made into 100g, mixed evenly with a ball mill, and then distributed for use.
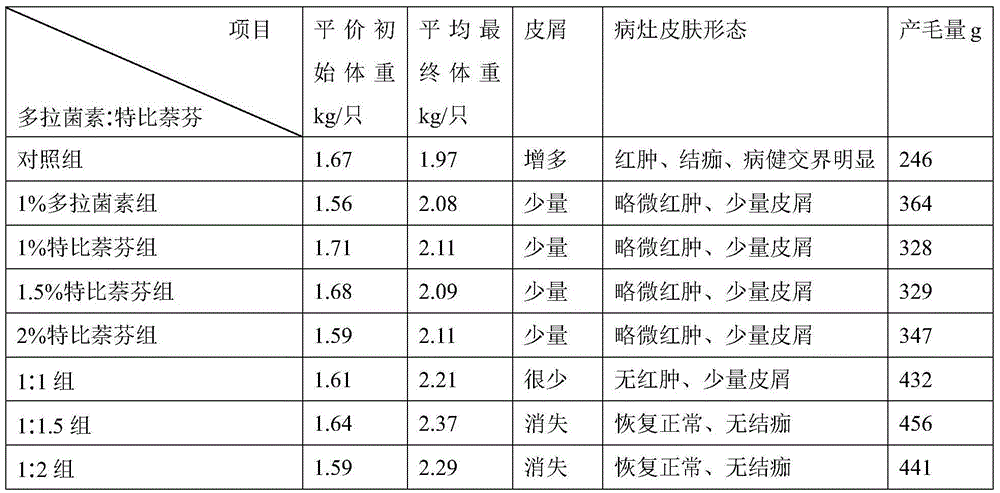
[0058] In order to prove the beneficial effect of the pharmaceutical composition of the present invention, the following test examples are provided.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com