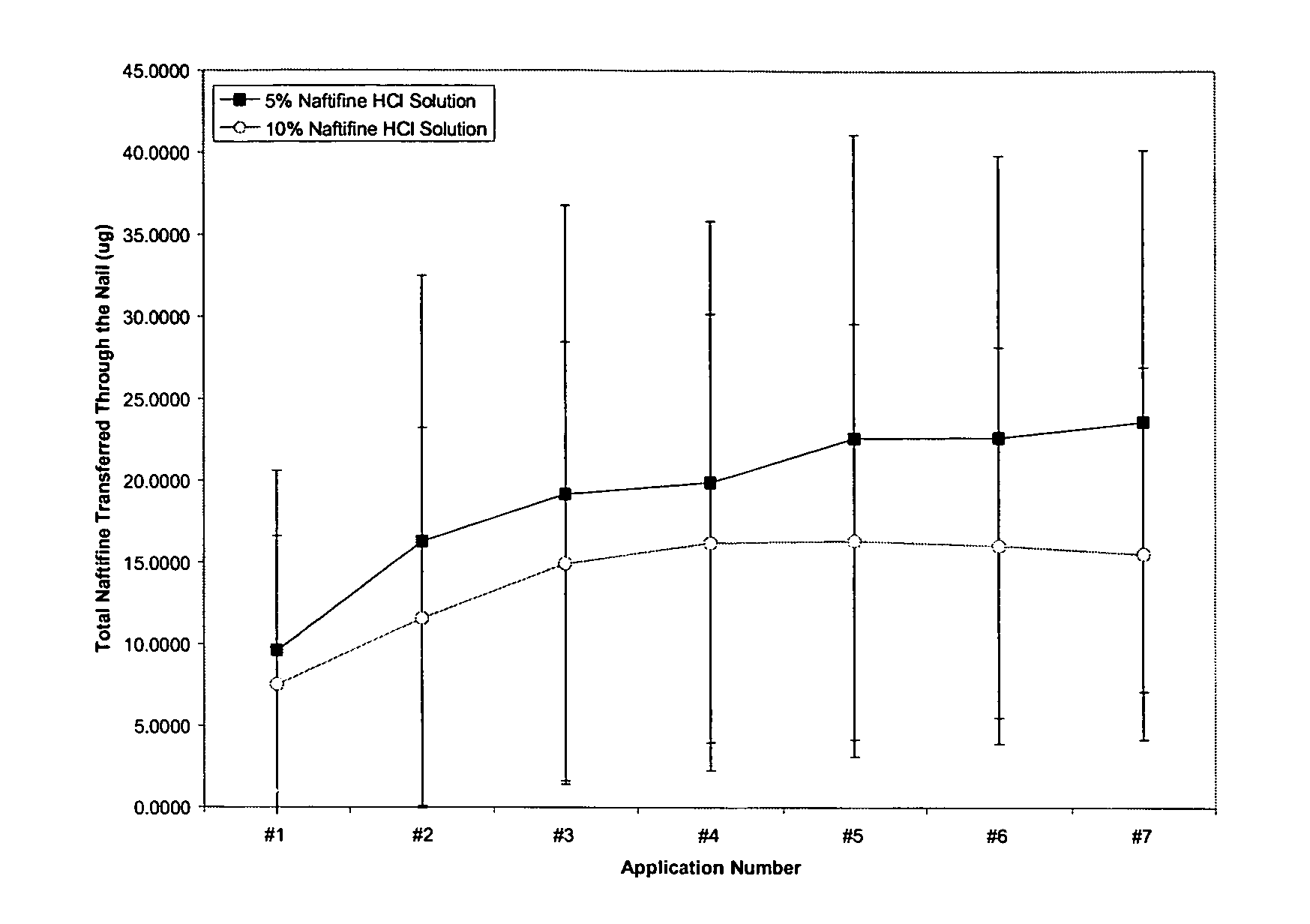
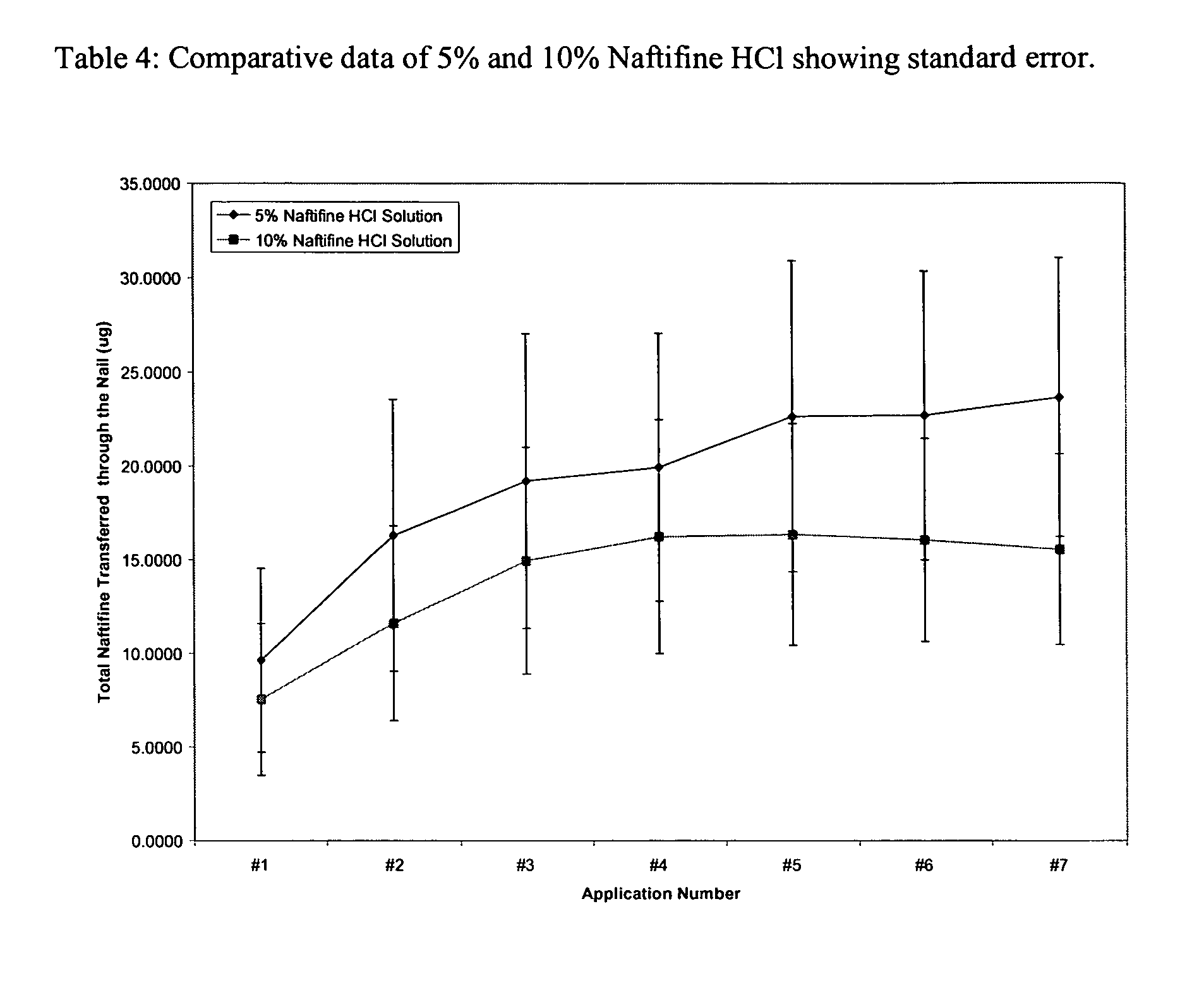
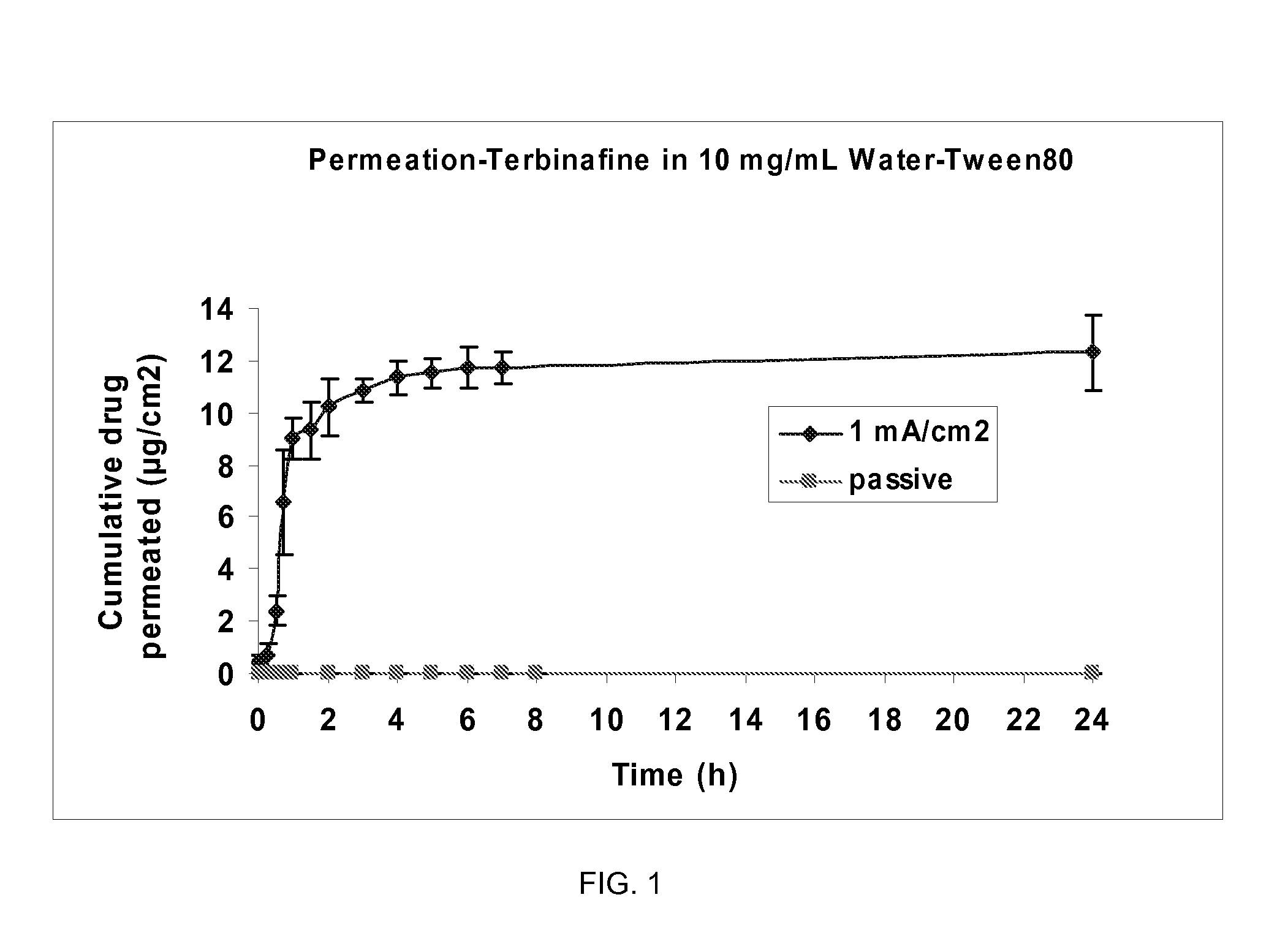
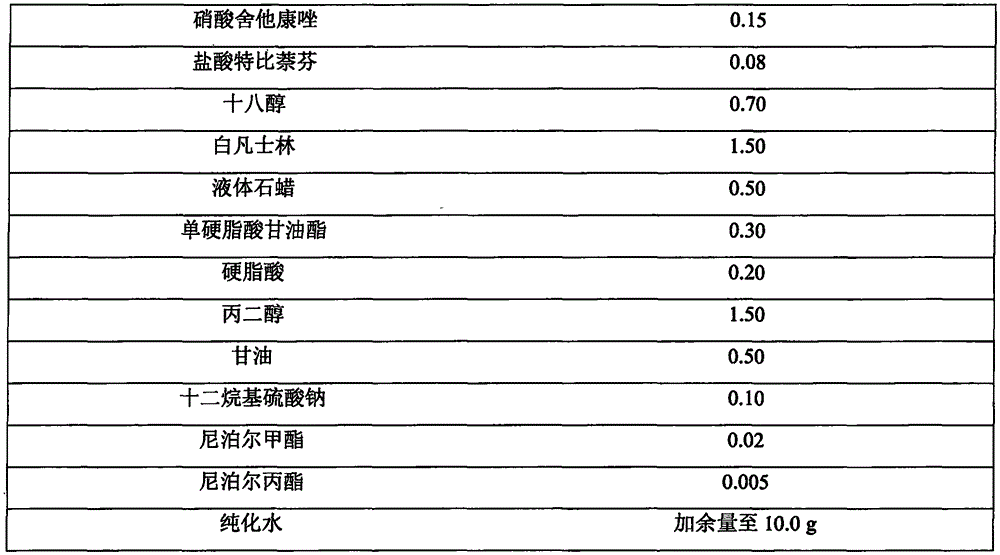
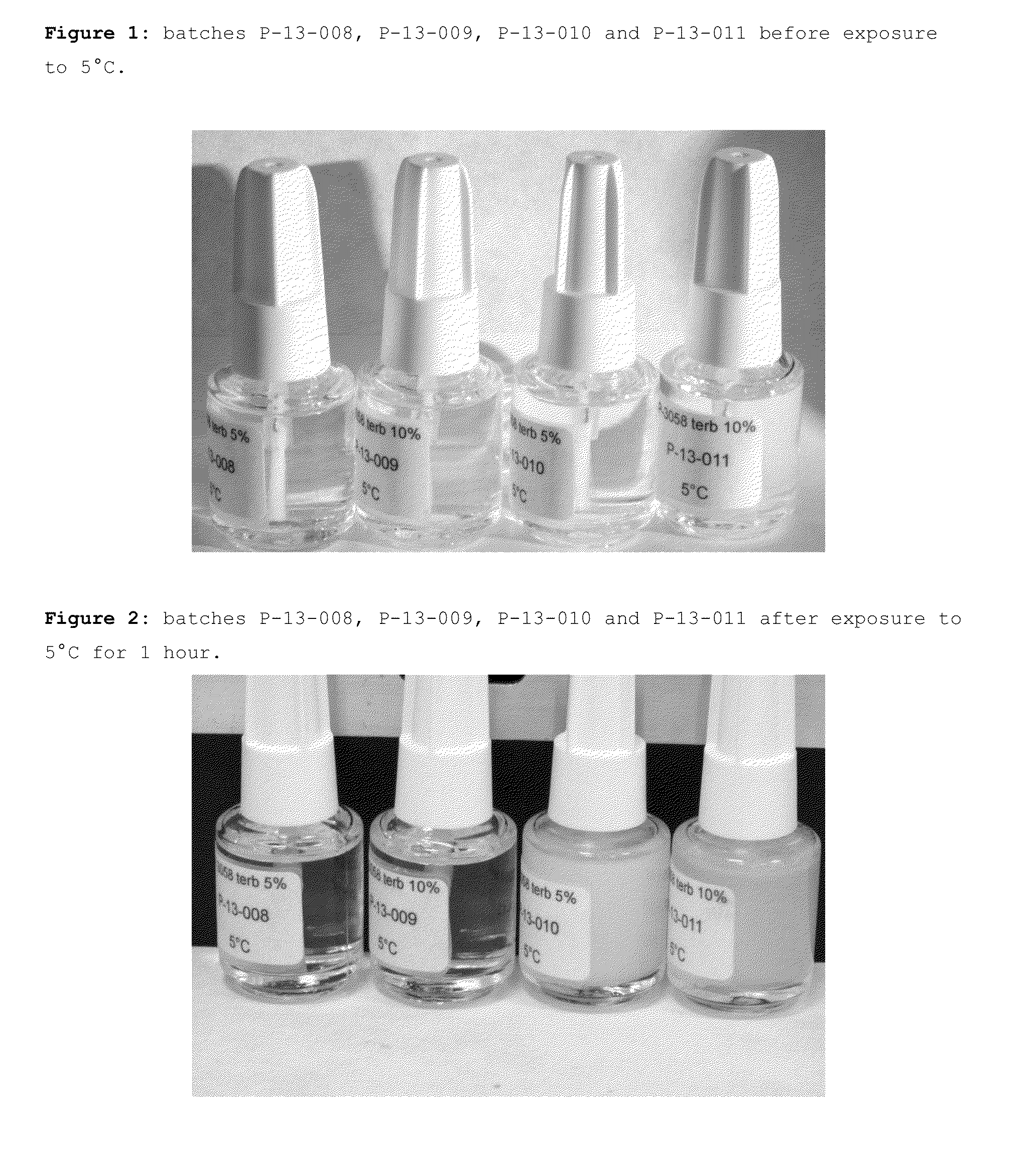
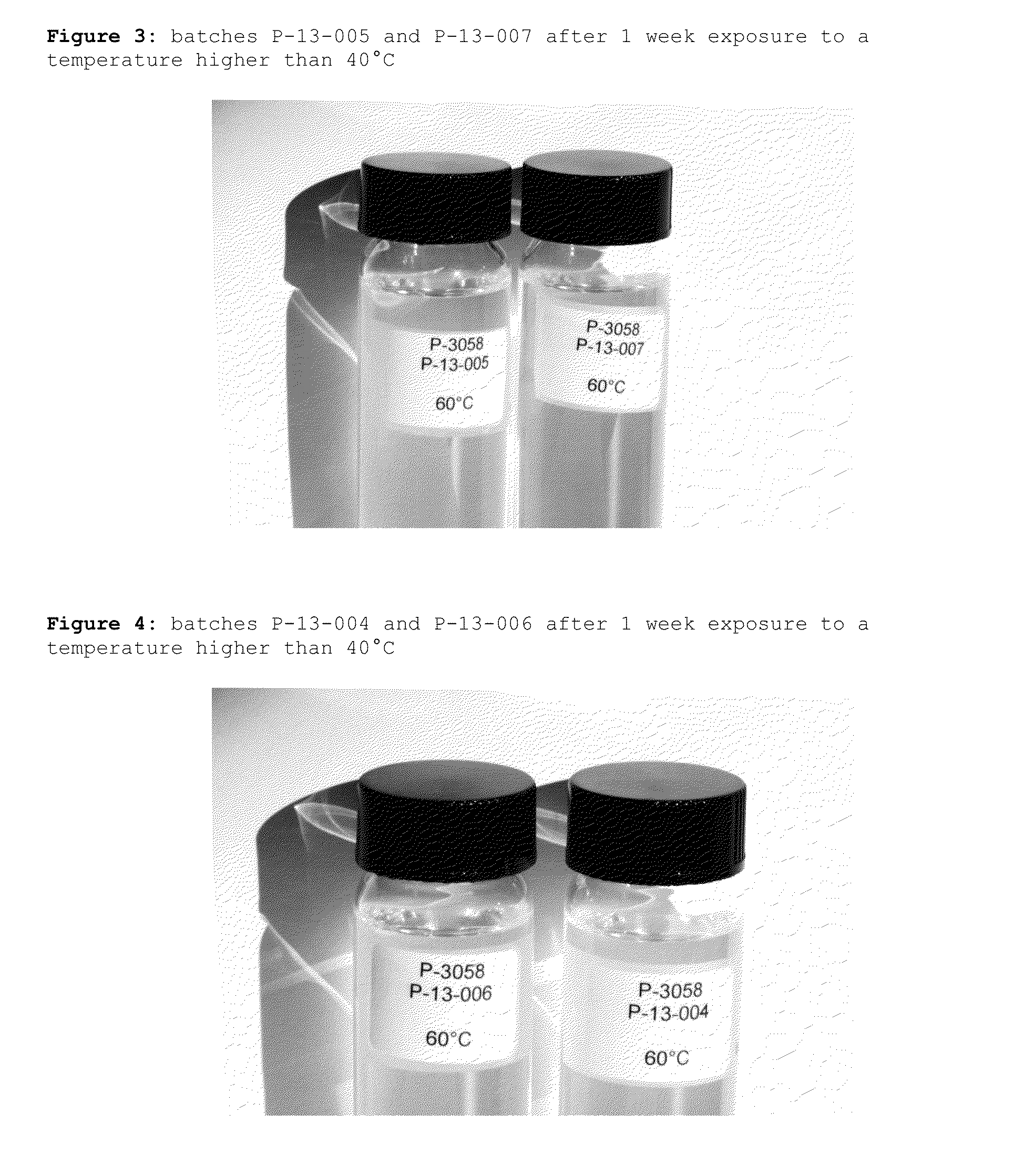
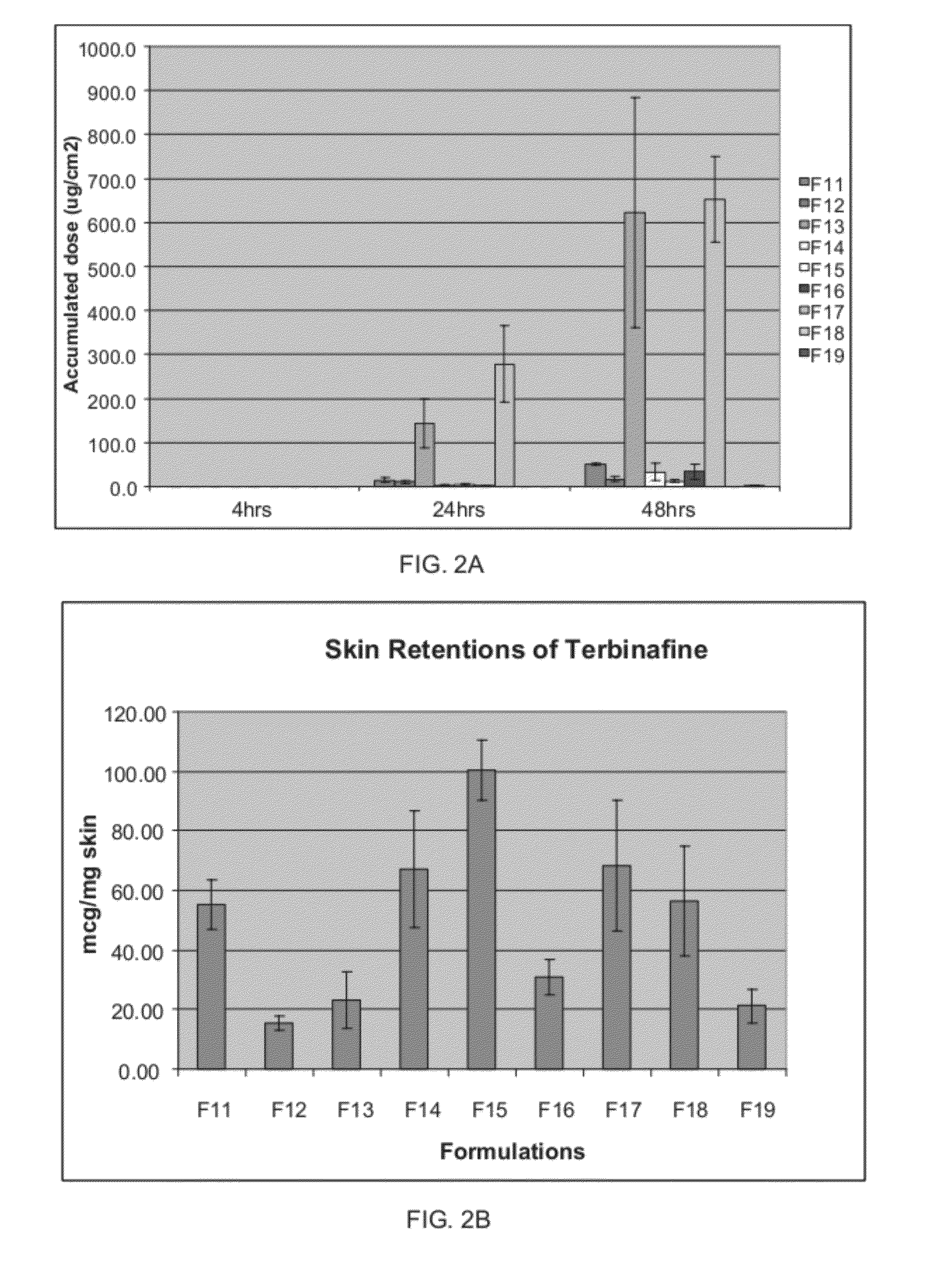
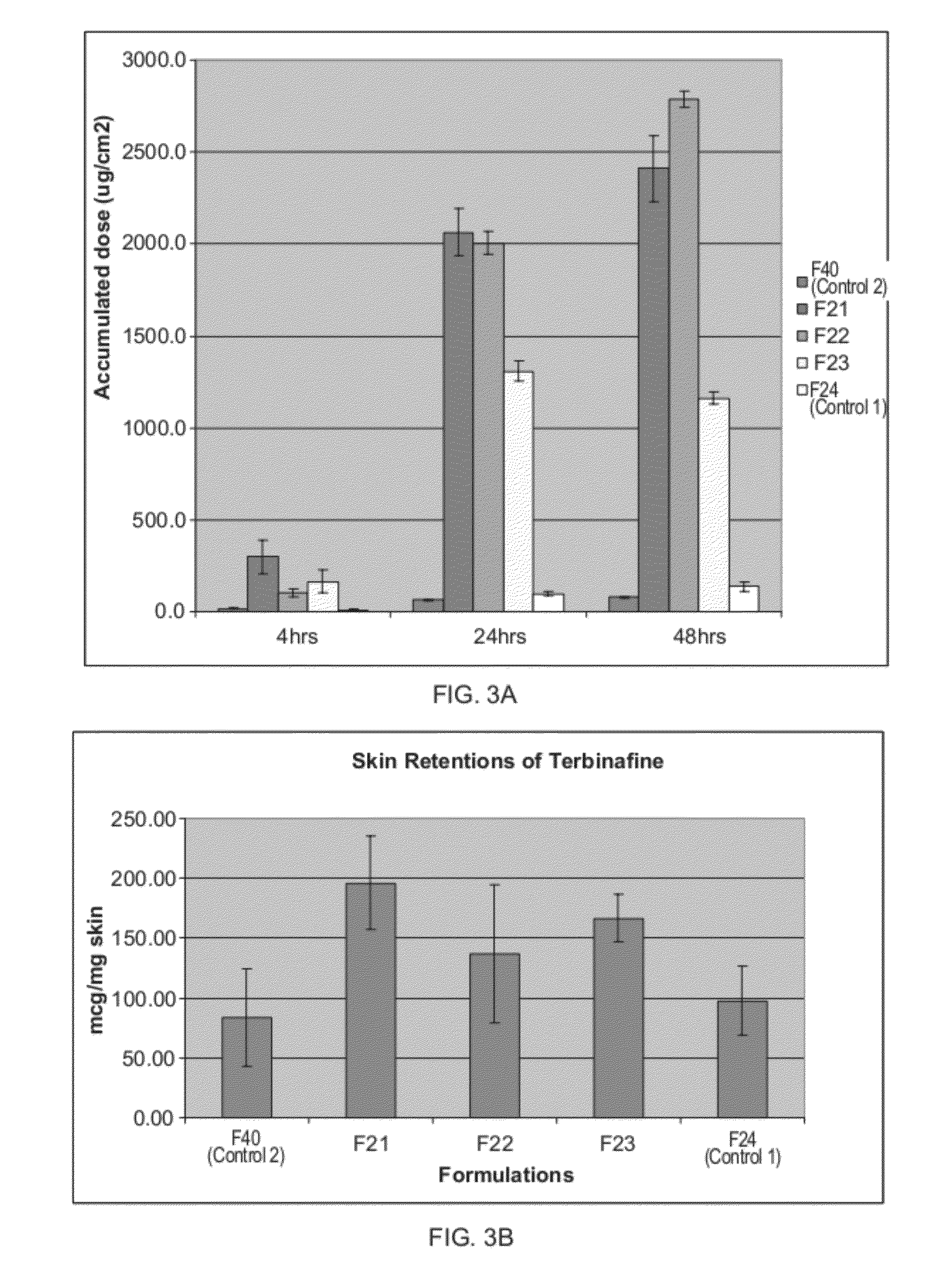
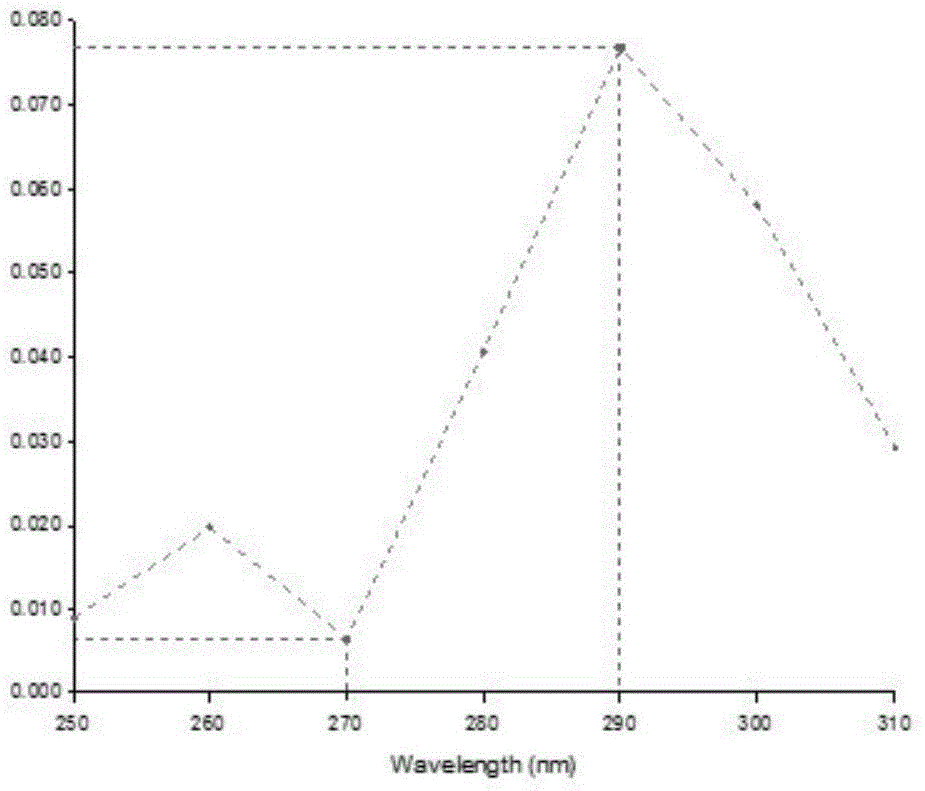
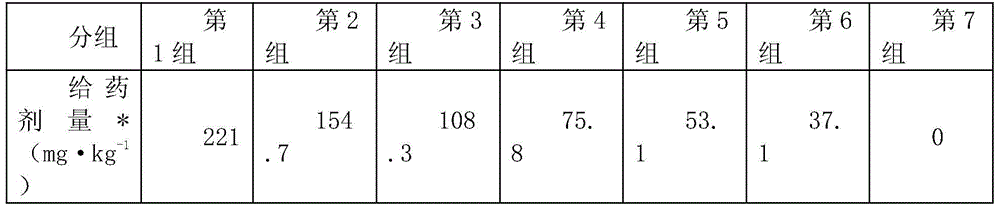
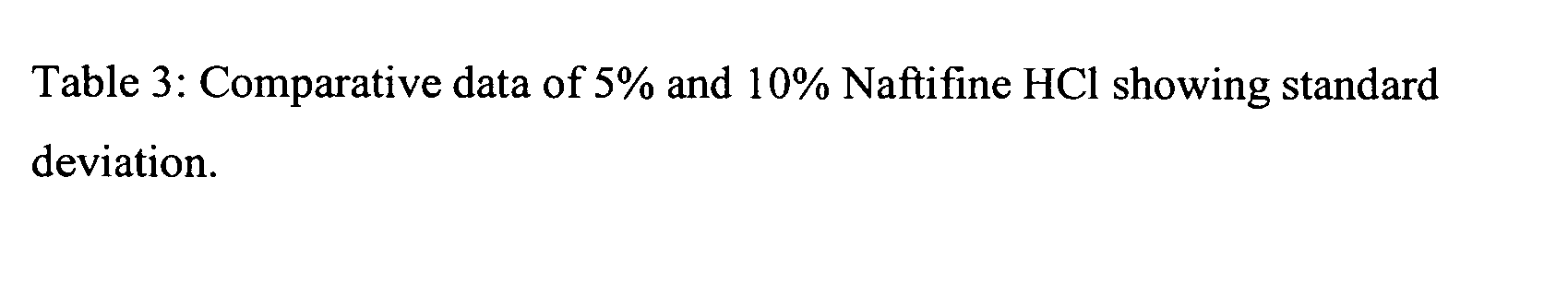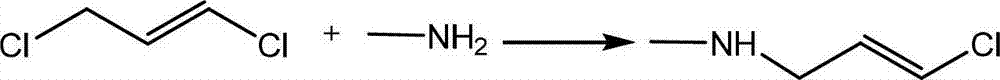Patents
Literature
64 results about "Hydrochloride terbinafine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
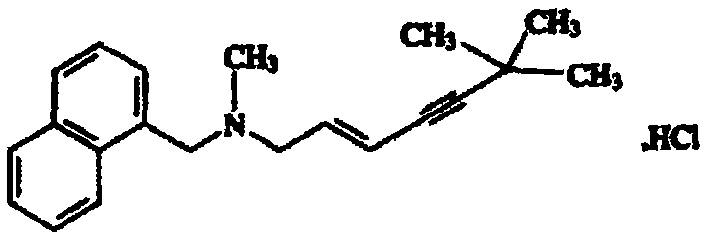
Terbinafine hydrochloride is a white fine crystalline powder that is freely soluble in methanol and dichloromethane, soluble in ethanol, and slightly soluble in water. It is highly hydrophobic and tends to accumulate in hair, skin, nails, and fatty tissue.
Pharmaceutical composition applicable to body tissue
The present invention provides a non-water soluble, film-forming composition which adheres to body tissue and forms a pharmaceutical carrier to provide localized delivery of an antifungal agent to a treatment site. The composition will typically include: (a) an alkyl cellulose; (b) a hydroxyalkyl cellulose; (c) a pharmaceutically acceptable polar protic solvent; (d) an antifungal agent selected from the group of naftifine, ciclopirox, terbinafine, pharmaceutically acceptable salts thereof, and combinations thereof; (e) an glycol ether; (f) an antipruritic agent selected from the group of camphor, menthol, butamben picrate, metacresol, benzyl alcohol, camphorated metacresol, juniper tar, phenol, phenolate sodium, resorcinol, camphorated metacresol, carbolic acid, and combinations,; and (g) a solubility enhancing agent, a surfactant, a wetting agent, or a combination thereof. The present invention also provides for the use of the composition composition of the present invention, in treating a fungal infection (e.g., nail fungus) in a mammal afflicted with such an infection.
Owner:QLT USA INC
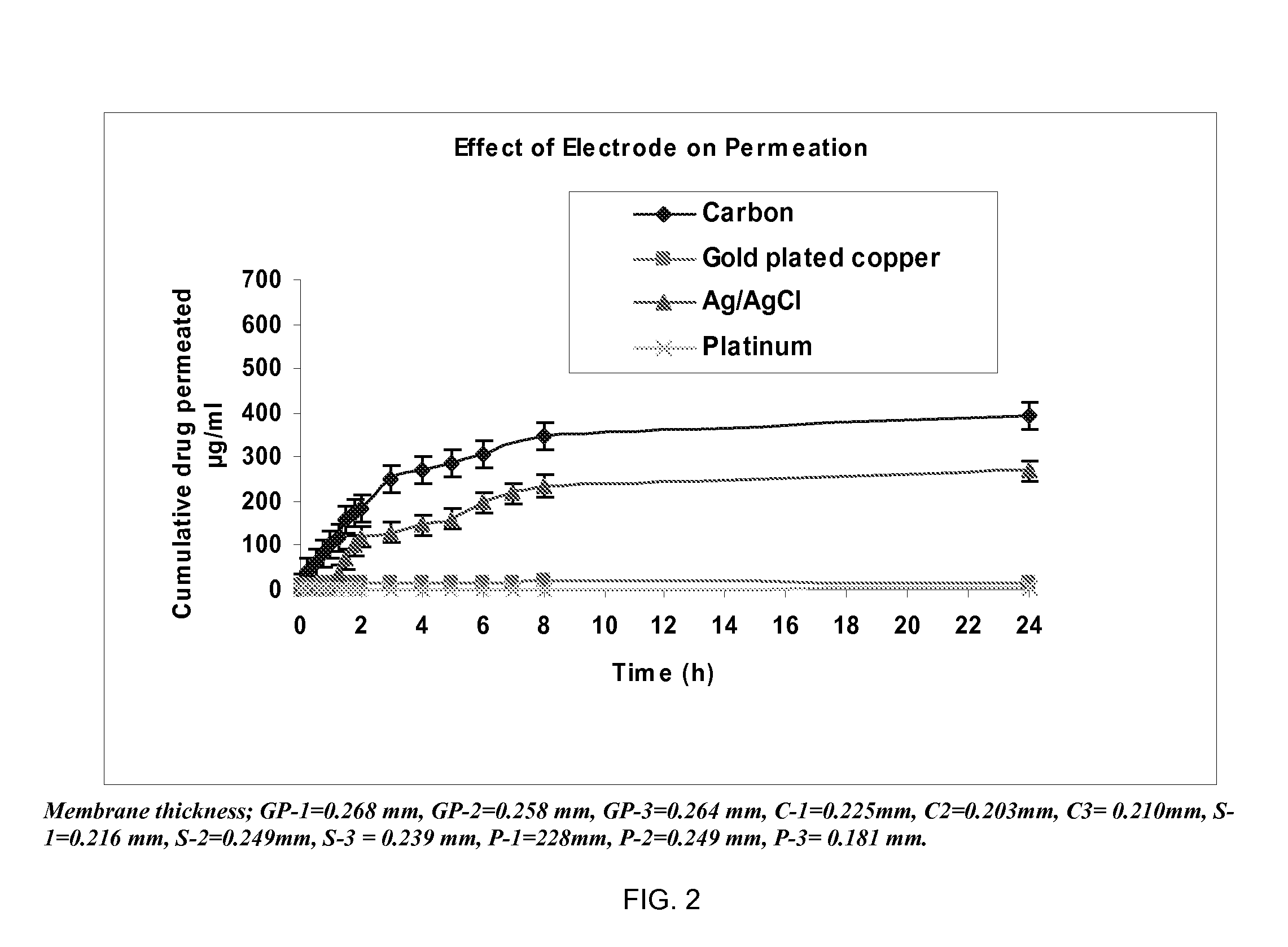
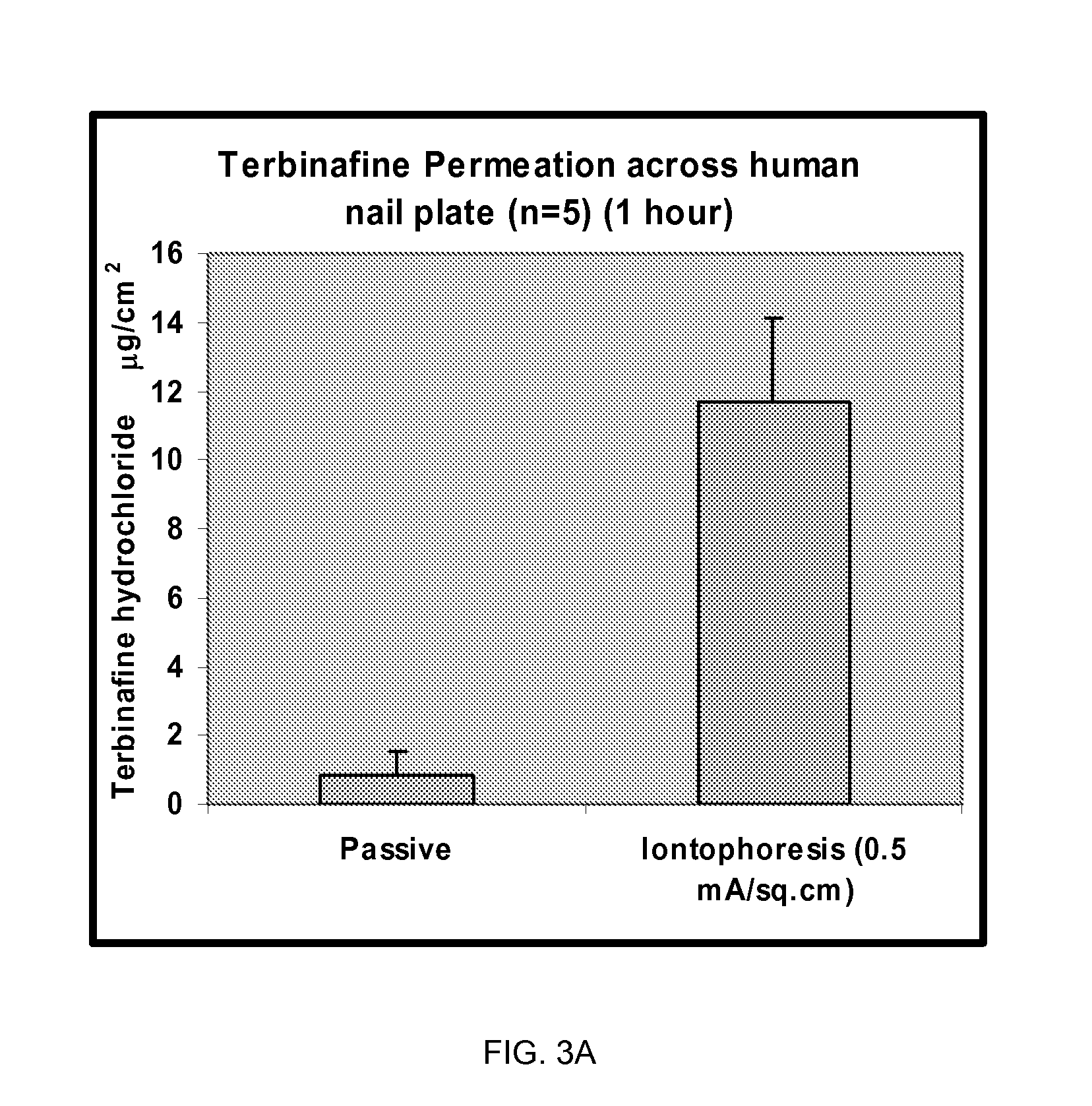
Pharmaceutical formulations for iontophoretic delivery of an Anti-fungal drug
InactiveUS20080261986A1Reduce deliveryEnhanced iontophoretic deliveryBiocideAntimycoticsAntifungalPharmaceutical formulation
Pharmaceutical formulations suitable for iontophoresis thereof that provide enhanced iontophoretic delivery of an anti-fungal drug to at least one body surface are described. Also described are pharmaceutical formulations suitable for iontophoresis comprising terbinafine and methods for administering terbinafine to a body surface via iontophoresis. In one embodiment, the body surface includes a nail plate and / or the skin.
Owner:NITRIC BIOTHERAPEUTICS INC
Antifungal drug delivery
An improved antifungal composition for topical application to the skin and nails comprises: (1) an allylamine antifungal compound; (2) an aliphatic alcohol substituted with an aromatic substituent in which the allylamine antifungal compound is soluble to a degree that a therapeutically effective concentration of the allylamine antifungal compound can be applied topically in solution; (3) a lower aliphatic alcohol in which the aromatic alcohol is soluble; and (d) water or a water-compatible solvent mixture. The allylamine antifungal compound can be terbinafine or naftifine. The aliphatic alcohol substituted with an aromatic substituent can be benzyl alcohol or phenethyl alcohol. The lower aliphatic alcohol can be ethyl alcohol or isopropyl alcohol. In an alternative, the composition can further comprise an additional antifungal compound. Another aspect of the invention is a method for treatment of a fungal infection of skin or nails comprising administering the antifungal composition of the present invention topically to the skin or nails in an amount therapeutically effective to treat the fungal infection.
Owner:TRANSPHASE
Antifungal drug composition for external use
InactiveCN106806368ASignificant effectPromote absorptionOrganic active ingredientsAntimycoticsRegimenTinea unguium
The invention provides an antifungal drug composition for external use, and a preparation method of an external preparation, and application of the composition in treating superficial fungal infections, such as tinea manus and pedis, tinea corporis, tinea cruris, tinea unguium, tinea versicolor and oidiomycosis cutis. The drug composition contains terbinafine or pharmaceutical salt thereof, sertaconazole or pharmaceutical salt thereof, and any one or multiple pharmaceutically acceptable excipient. An experimental study shows that the terbinafine or pharmaceutical salt thereof and the sertaconazole or pharmaceutical salt thereof have obvious synergistic effect when the proportion thereof in the drug composition for treating the superficial fungal infections is (1: 10) to (5: 1)(w / w). The antifungal drug composition for treating the superficial fungal infections has the advantages of being good in security, excellent in curative effect, short in treatment course, high in curing rate, low in relapse rate and the like.
Owner:BEIJING ENCHENG KANGTAI BIOLOGICAL TECH
Local anti-infective agent for treatment of nail fungal infections
InactiveUS20080193508A1Enhanced topical deliveryEnsure penetrationBiocideCosmetic preparationsBenzoic acidAntifungal
Owner:JUVENTIO
Topical antifungal treatment
The present invention is a topical skin preparation for treatment of fungal infections of the skin and nails. The preparation comprises triacetin in combination with an antifungal agent. In a preferred form, the preparation further comprises, a fatty acid source such as a fish oil. In a preferred embodiment, cod liver oil and tolnaftate are used in combination with triacetin. The concentrations of these constituents are 96.0-99.0% concentration triacetin, 0.5-3.0% concentration tolnaftate and 0.5-1.0% concentration cod liver oil, in one preferred embodiment. Other compounds, such as ethyl alcohol, amino acids such as n-acetylcysteine, and herbal additives may also be added to the preparation. Further, other antifungal agents such as nystatin, clortimazole, econazole, ketoconazole, miconazole, solconazole, oxiconizole, naftifine, terbinafine, and butenafine, for example, may be substituted for the antifungal agent tolnaftate. The preparation of the present invention is effective in treating immune compromised patients and those with diabetes, as well as relatively healthy persons.
Owner:BOMMARITO ALEXANDER A
Method of treating onychomycosis
The present invention is directed to a nail lacquer consisting essentially of terbinafine as an antimycotic agent, hydroxypropyl chitosan as film forming agent, water and a lower alkanol as solvents. The invention is also directed to a method for treating onychomycosis by topically administering such a nail lacquer to a patient in need of such a treatment.
Owner:POLICHEM SA
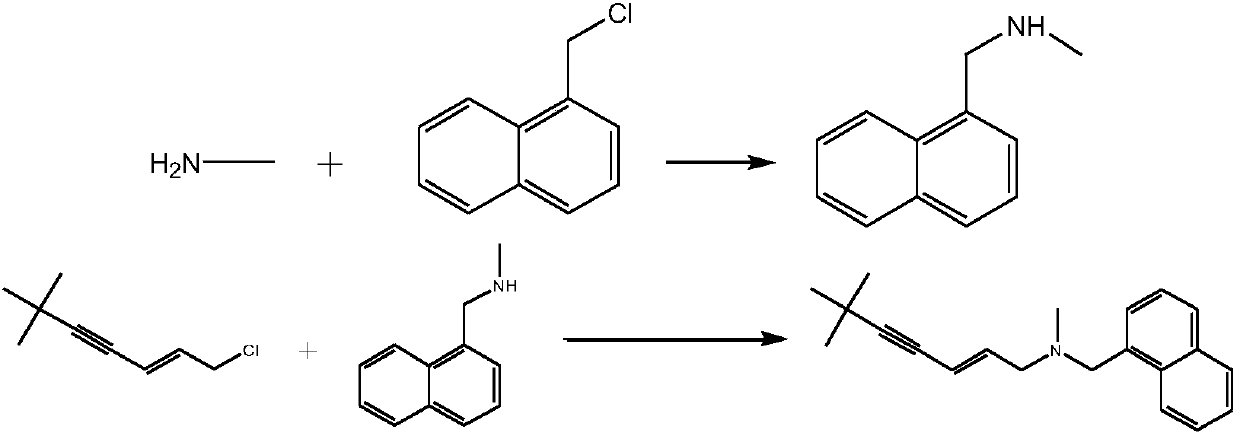
Synthetic method of terbinafine
ActiveCN108017544AHigh yieldOvercome yieldAmino preparation by functional substitutionOrganic layerAlkyne
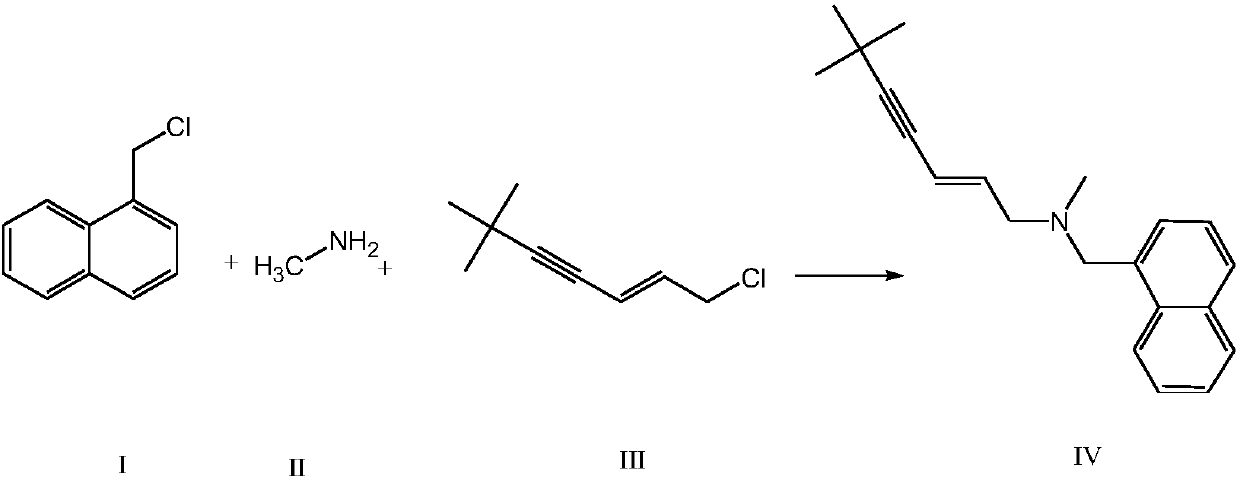
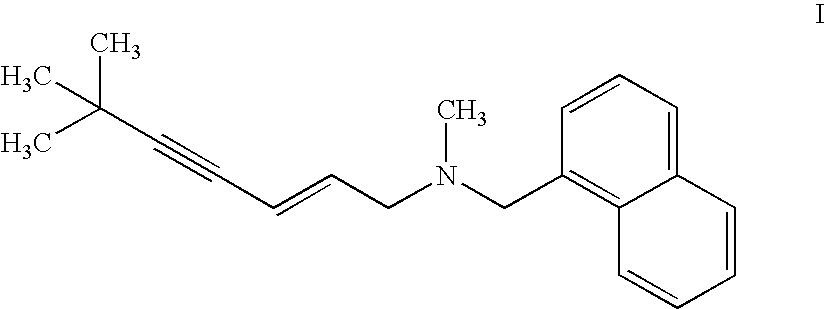
The invention provides a synthetic method of terbinafine. Monomethylamine, 1-chloromethyl naphthalene and 1-chloro-6,6-dimethyl-2-heptene-4-alkyne are subjected to a one-step reaction to generate theterbinafine. Particularly, the monomethylamine is slowly added into a solvent, and then an acid-binding agent is added; the 1-chloromethyl naphthalene and the 1-chloro-6,6-dimethyl-2-heptene-4-alkyneare simultaneously slowly dropwise added, and a dropwise adding speed is controlled so as to enable both the 1-chloromethyl naphthalene and the 1-chloro-6,6-dimethyl-2-heptene-4-alkyne to be simultaneously dropwise added up; a temperature control reaction is performed for 2 to 3 hours at a temperature of 10 to 20 DEG C; chloroform is added to extract reaction liquid, vacuum concentration is carried out on an organic layer to a dry state, and ethyl acetate is added into residues to carry out crystallization to obtain the terbinafine. The synthetic method is high in yield, simple in step and convenient to operate, generates few byproducts, and is beneficial to industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Topical antifungal treatment
The present invention is a topical skin preparation for treatment of fungal infections of the skin and nails. The preparation comprises triacetin in combination with one or more antifungal agents. In a preferred form, the preparation comprises a combination of the antifungal agents tolnaftate and grisiofulvin are used in combination with triacetin. The concentrations of these constituents are 40 to 50% concentration triacetin, 30 to 50% acetone, 0.5-3.0% concentration tolnaftate and 0.5-4.0% concentration grisiofulvin, in one preferred embodiment. Other compounds, such as ethyl alcohol, acetone, amino acids such as n-acetylcysteine, and herbal additives may also be added to the preparation. Further, other antifungal agents such as nystatin, clortimazole, econazole, ketoconazole, miconazole, solconazole, oxiconizole, naftifine, terbinafine, and butenafine, for example, may be substituted for the antifungal agents tolnaftate and grisiofulvin. The preparation of the present invention is effective in treating immune compromised patients and those with diabetes, as well as relatively healthy persons.
Owner:BOMMARITO ALEXANDER A
Medicine composition for treating skin diseases as well as preparation method and application thereof
InactiveCN104546885AQuick treatmentImprove efficiencyOrganic active ingredientsAntimycoticsDiseaseSide effect
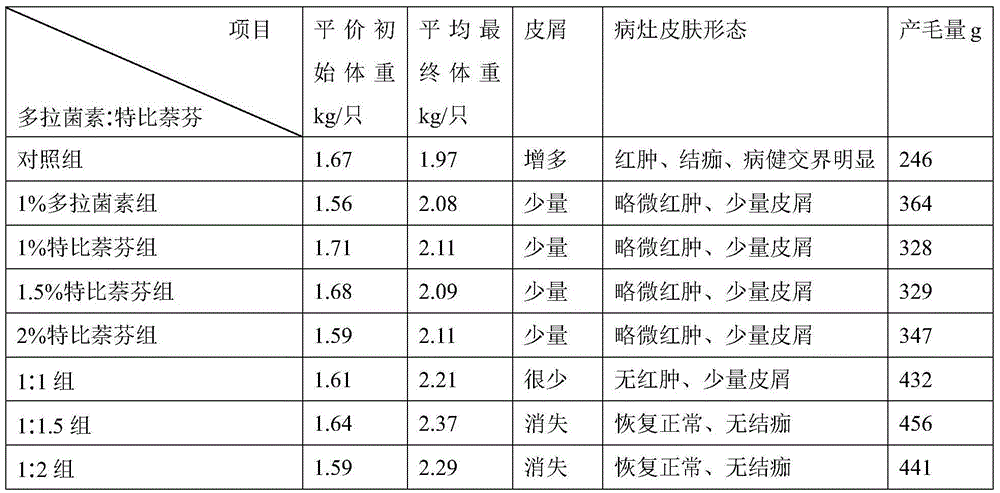
The invention discloses a medicine composition for treating skin diseases. The medicine composition contains the following raw material medicines in parts by weight: 1 part of doramectin and 1-2 parts of terbinafine. The invention further provides a preparation method for the medicine composition and an application of the medicine composition in preparation for a medicine for treating skin diseases. Pharmacological experiments indicate that the medicine composition disclosed by the invention is safe and free from toxic and side effects, fast to become effective and high in effective rate, low in medication dose and low in administration frequency, as well as wide in selective dosage forms, simple in administration mode, and suitable for large-scale clinic popularization and application.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Preparation method of terbinafine hydrochloride
ActiveCN102898314ASimplify production stepsRaw materials are easy to obtainAmino preparation by functional substitutionAqueous solutionAcetylene
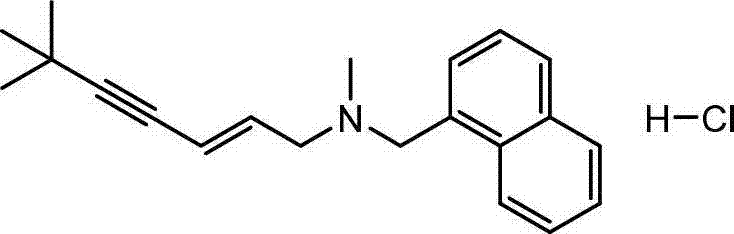
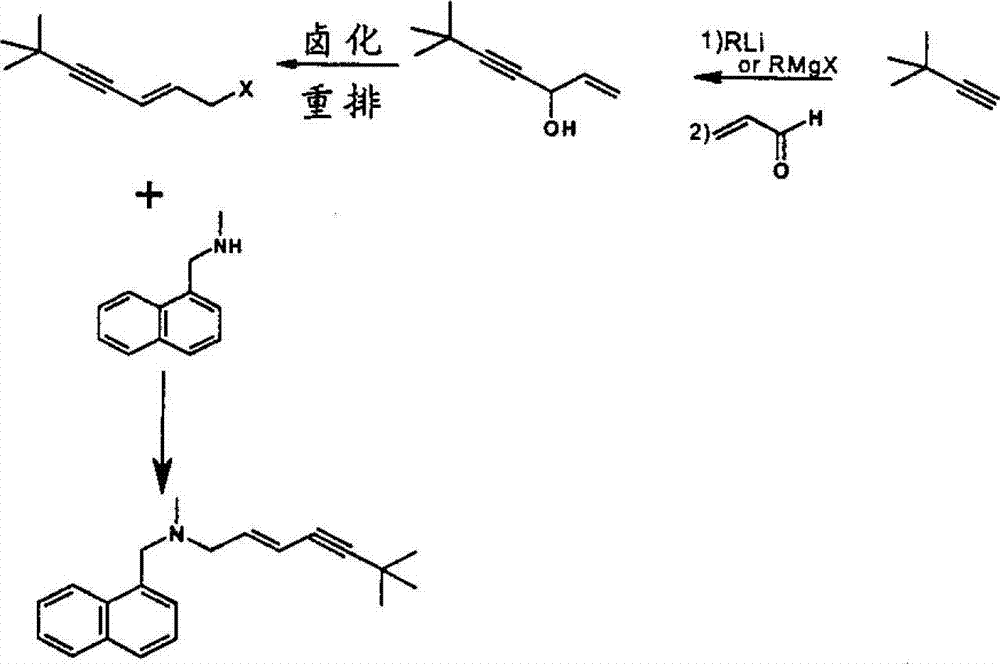
The invention discloses a preparation method of terbinafine hydrochloride, and belongs to the technical field of medicine. According to the method, a monomethylamine aqueous solution and (E)-1, 3 dichloropropene are taken as materials to react to obtain (E)-1-methylamino-3-chlorine-propylene; then (E)-1-methylamino-3-chlorine-propylene reacts with tertiary butyl acetylene to obtain (E)-N-(6, 6-dimethyl-2-heptylene-4-alkynyl) methylamine; and then the (E)-N-(6, 6-dimethyl-2-heptylene-4-alkynyl) methylamine reacts with 1-chlorine-methylnaphthalene to obtain terbinafine, and hydrochloric acid is added for salification to obtain terbinafine hydrochloride. According to the method, materials are easy to get, the cost is low, the preparation method is simple, the yield is high, and the method is suitable for industrial production.
Owner:山东艾兰药业有限公司
Compound terbinafine emulsifiable paste and preparation method thereof
InactiveCN101181275AStrong medicineQuick effectOrganic active ingredientsAntimycoticsRegimenSkin fungal infection
The invention discloses a terbinafine compound preparation for treating eczema, dermatitis, body ringworm, tinea cruris, tinea manus and pedis and the preparation method; the invention mainly uses terbinafine hydrochloride and clobetasol propionate or triamcinolone acetonide acetate, which is further matched with O / W emulsion matrix for the preparation of cream. The invention is characterized by fast onset of action, short treatment course and low recurrence rate for treating skin fungal infection with local inflammatory or chronic eczema.
Owner:林华清
Methods of administering topical antifungal formulations for the treatment of fungal infections
InactiveUS20100086504A1RiskLoss of feelingCosmetic preparationsBiocideLipid formationSURFACTANT BLEND
The present invention relates to topical antifungal formulations comprising one or more antifungal (e.g., terbinafine), a lipid and a surfactant, and uses thereof for the treatment of skin and nail fungal infections.
Owner:TDT
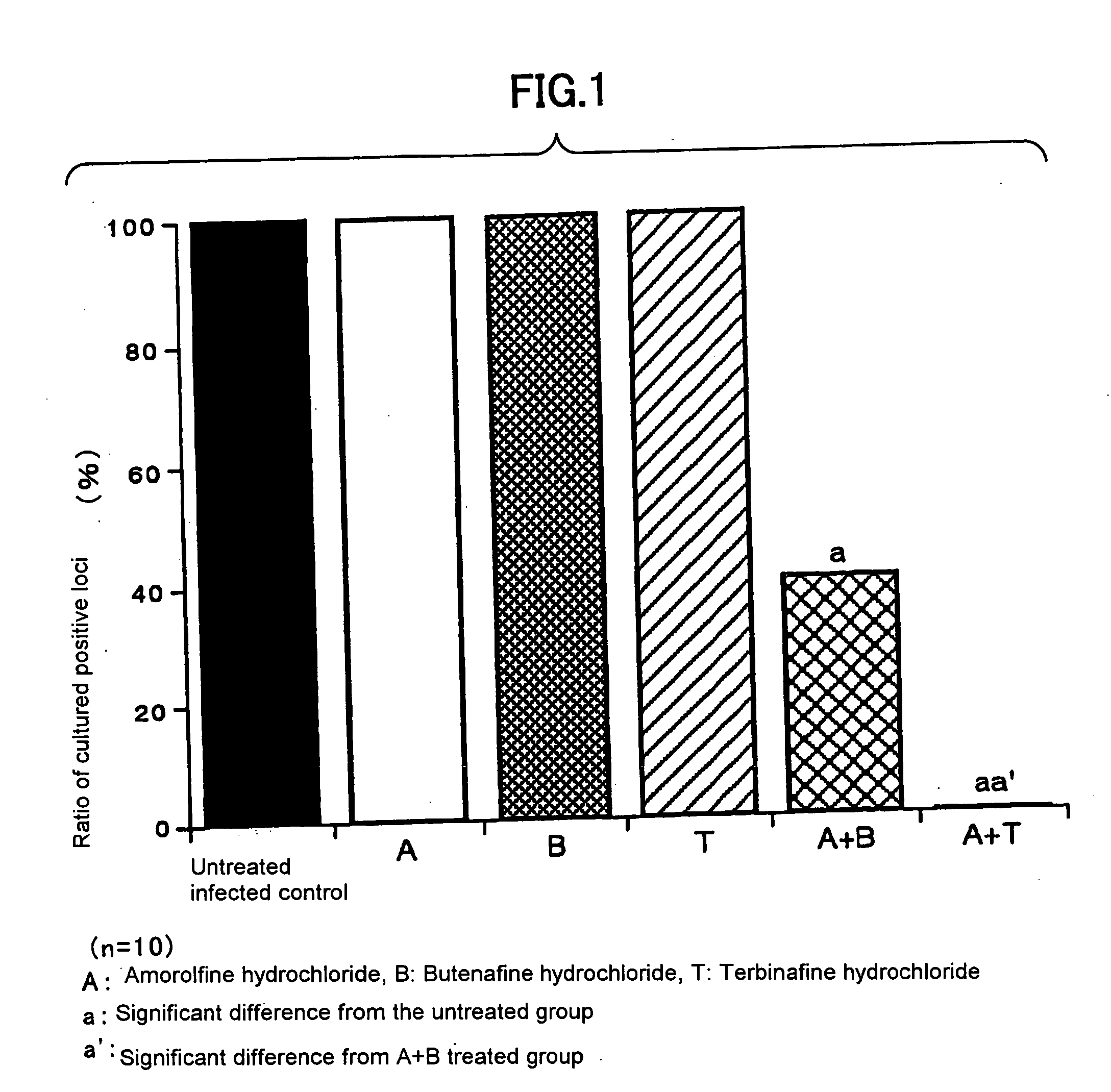
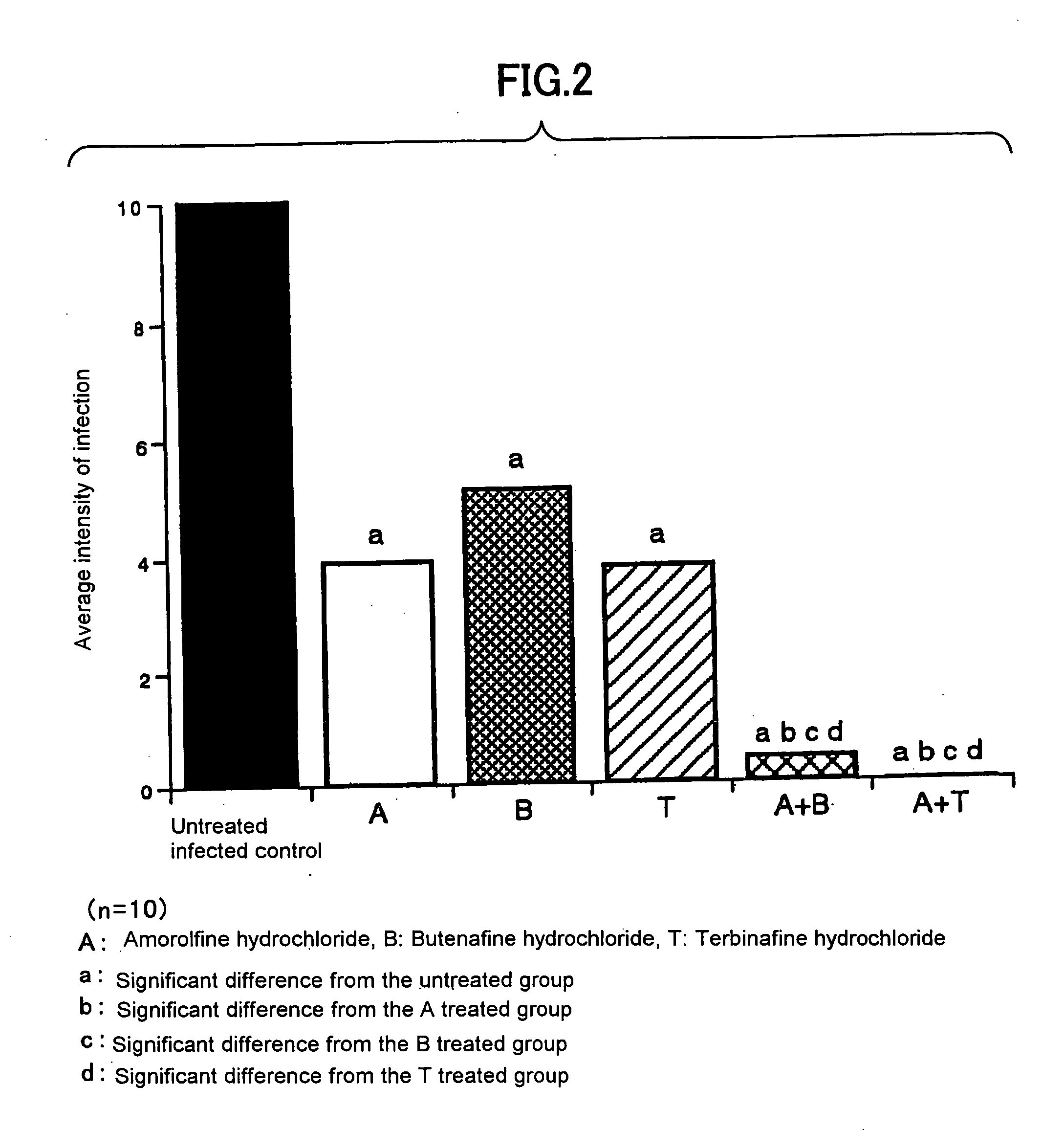
Antifungal agent for topical use
InactiveUS20050113371A1Good effectImprove antifungal effectOrganic active ingredientsBiocideBeriberiTopical uses
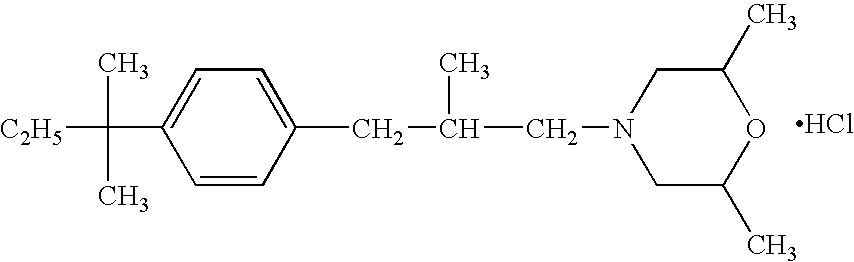
The present invention provides an antifungal agent for topical use and remedy for athlete's foot that comprises a new combination of medicines and has a strong antifungal activity against both genus Trichophyton and genus Candida. The antifungal agent for topical use and remedy for athlete's foot contain amorolfine with butenafine or terbinafine.
Owner:SATO PHARMA
Nail patch
The present subject matter provides a nail patch comprising a backing layer and a pressure-sensitive adhesive layer disposed on at least one side of the backing layer, wherein the pressure-sensitive adhesive layer comprises a pressure-sensitive adhesive base, terbinafine and / or a pharmacologically acceptable salt thereof, and sodium acetate and / or sorbitan monolaurate as a solubilizer.
Owner:HISAMITSU PHARM CO INC
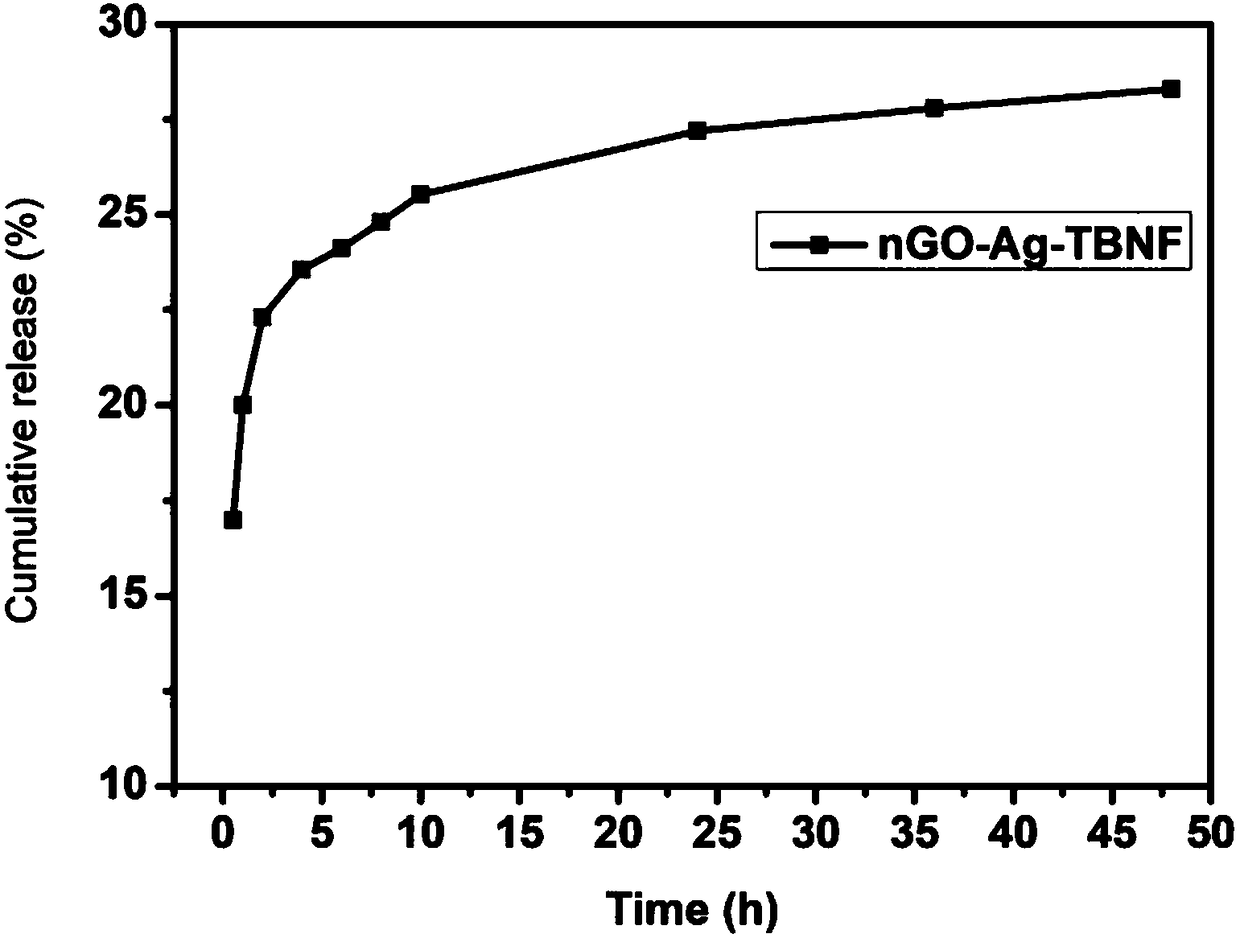
Graphite oxide nano silver terbinafine antifungal sustained-release thermosensitive hydrogel, and preparation method and application of hydrogel
ActiveCN108158975AFacilitated releaseExtension of timeOrganic active ingredientsAntimycoticsAntifungalAdjuvant
The invention provides graphite oxide nano silver terbinafine antifungal sustained-release thermosensitive hydrogel, and a preparation method and an application of the hydrogel. The graphite oxide nano silver terbinafine antifungal sustained-release thermosensitive hydrogel takes graphite oxide as a drug sustained-release carrier, so that the drug release and action time is prolonged; at the sametime, loaded nano silver can continuously release silver ions to assist in improving an antifungal effect; due to a special sterilization mechanism of the silver ions, a drug resistance problem is notgenerated; the recurrence probability is low; similarly, poloxamer serves as an adjuvant; a drug can be in a liquid state at the room temperature; when the drug is applied to an affected site, the drug is changed into the hydrogel due to temperature rise; the administration is convenient; the contact time of the drug with the affected site can be prolonged; in addition, since the drug exists as anano ingredient, part of the drug can permeate into skin pores in a liquid form and is changed into the hydrogel due to an effect of the body temperature; and the fungicidal action time can be further prolonged.
Owner:JILIN UNIV
Tinea resisting cream
InactiveCN1634108AAchieve bacteriostasisAchieve bactericidalOrganic active ingredientsAntimycoticsDiseaseRoxithromycin
Disclosed is a tinea resisting cream which is used for treating diseases caused by shadow position fungus infection, such as tinea corporis, tinea cruris, extremity corporis. The medicament comprises a dozen of constituents including lauryl alcohol, terbinafine, ethyl p-hydroxybenzoate, Roxithromycin, and methyl ethylene glycol.
Owner:秦现杰
Nail patch
The present subject matter provides a nail patch comprising a backing layer and a pressure-sensitive adhesive layer disposed on at least one side of the backing layer, wherein the pressure-sensitive adhesive layer comprises a pressure-sensitive adhesive base, terbinafine and / or a pharmacologically acceptable salt thereof, and sodium acetate and / or sorbitan monolaurate as a solubilizer.
Owner:HISAMITSU PHARM CO INC
Terbinafine or its salt film forming gel composition and uses thereof
ActiveCN101138556AGood flexibilitySmall molecular weightOrganic active ingredientsAntimycoticsCelluloseCross-link
The present invention discloses a compound of an Terbinafine or the halo-Terbinafine membrane forming gel, which comprises the components of the following weight percentages comprising 0.5 percentage to 7 percentage of alkyl cellulose, 1 percentage to 10 percentage of etherifying agent, 0.5 percentage to 5 percentage of cross linking agent, 0.5 percentage to 5 percentage of accentuator, 75 percentage to 90 percentage of dissolvant0.1 percentage to 5 percentage, of Terbinafine or the halo-Terbinafine. The cross linking agent is the saturated fat or the alcoholic acid, the chemical general formula of which is CnH2n+2-m-l(OH)m(COOH)l. Among the formula, m or n or 1 is the integer and the n is no less than m, which is no less than 2. 1 is no less than 0 and the result of m plus 1 is 4 to 8. The result of n plus 1 is 4 to 8. In the present invention, the compound of the Terbinafine or the halo-Terbinafine local membrane forming gel is applied for treatment of the local fungus inflammation. A water drain protective membrane with smooth property, firm property, wear-resisting property and duration property is formed on the surface of the ulcer when the present invention is applied. Compared with all kinds of formulations of the Terbinafine or the halo-Terbinafine in the present technique, the membrane formed by the gel compound is not likely to experience fracture, dissolution or corrosion. The membrane has the properties of long maintenance time and slow releasing of the medicine.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Penetrating carrier, Anti-fungal composition using the same and method for treatment of dermatophytes
An antifungal liquid composition for topical administration is provided that contains turpentine and at least one of peppermint oil, mineral oil, and essential oil alcohols, and one or more antifungal medications selected from ciclopirox olamine, terbinafine, tolnaftate, microconazole, itraconazole, ketoconazole, econazole, and fluconazole, and optionally one or more antifungal essential oils, and its use in the topical treatment of fungal infection, particularly of the nail.
Owner:POWER PHARMA LLC
Adhesive patch for treatment of tinea unguium
Owner:HISAMITSU PHARM CO INC
Insecticide for animal
InactiveCN101428085ANo side effectsHas a fragranceOrganic active ingredientsAntiparasitic agentsDiseaseTreatment effect
The invention relates to an animal drug, in particular to a pesticide for animals, which belongs to an external used drug suitable for disinfestation for animals or curing the skin diseases for the animals. The invention adopts the solution that the pesticide includes mandarin oil, hesperetin with purity being more than and equal to 98.6 percent and terbinafine with purity being more than and equal to 99.8 percent, and the proportion among the mandarin oil, the hesperetin and the terbinafine is 100:1 to 2:0.25 to 0.9. During the manufacturing process, the hesperetin and the terbinafine are added into the mandarin oil in sequence according to the proportion, stirred at the normal temperature to be completely dissolved and uniformly mixed to obtain the finished product. The finished product is colorless or faint yellow transparent liquid, has the delicious taste of the orange, has good treatment effect to the skin disease, the fungus diseases, the acariasis and the acarid diseases of animals such as cats, dogs and the like, and has strong repelling and killing action to various mites, lice and flea.
Owner:TIANJIN SHENGJI GRP CO LTD
Liquid medicine for preventing and killing fleas
The invention discloses liquid medicine for preventing and killing fleas, and belongs to the technical field of epidemic prevention. The liquid medicine is prepared from, by weight, 5-50 parts of rhizoma atractylodis, 10-80 parts of coptis chinensis, 1-4 parts of terbinafine, 1-4 parts of ketoconazole, 1-4 parts of abamectin, 1-4 parts of Emma rhzomorph, 1-4 parts of eprinomectin, 1-4 parts of essential oil, 50-200 parts of garlic and 200-800 parts of brown sugar. The liquid medicine is safe and convenient to use, nontoxic to human and animal, free of pollution and capable of effectively preventing and killing fleas and louse parasitizing on pet cats and dogs, and the death rate of the fleas is large.
Owner:黄晓
Antifungal drug delivery
An improved antifungal composition for topical application to the skin and nails comprises: (1) an allylamine antifungal compound; (2) an aliphatic alcohol substituted with an aromatic substituent in which the allylamine antifungal compound is soluble to a degree that a therapeutically effective concentration of the allylamine antifungal compound can be applied topically in solution; (3) a lower aliphatic alcohol in which the aromatic alcohol is soluble; and (d) water or a water-compatible solvent mixture. The allylamine antifungal compound can be terbinafine or naftifine. The aliphatic alcohol substituted with an aromatic substituent can be benzyl alcohol or phenethyl alcohol. The lower aliphatic alcohol can be ethyl alcohol or isopropyl alcohol. In an alternative, the composition can further comprise an additional antifungal compound. Another aspect of the invention is a method for treatment of a fungal infection of skin or nails comprising administering the antifungal composition of the present invention topically to the skin or nails in an amount therapeutically effective to treat the fungal infection.
Owner:马塞尔・尼姆尼 +1
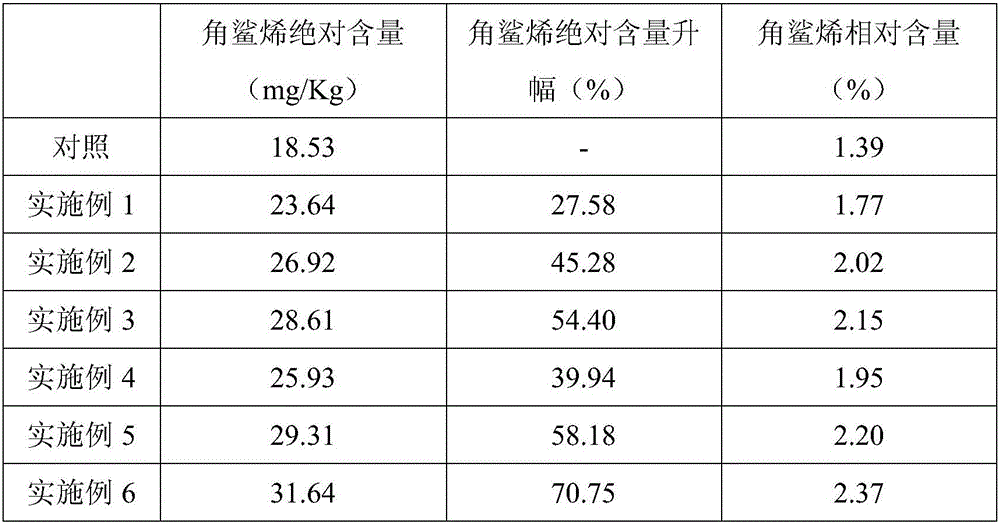
Terbinafine preparation and applications of terbinafine preparation in tobacco leaf squalene content improvement
InactiveCN106106449AIncrease contentHigh economic valueBiocidePlant growth regulatorsNicotiana tabacumPhosphate
The invention discloses a terbinafine preparation and applications of the terbinafine preparation in tobacco leaf squalene content improvement, wherein the terbinafine preparation comprises, by weight, 10-20% of terbinafine, 5-8% of tris(dodecyl alcohol polyether-4)phosphate, and the balance of ethanol. According to the present invention, the terbinafine preparation is added during the tobacco growth process, the squalene content in the tobacco leaf can be increased, and the economic value of the tobacco leaf is improved.
Owner:CHINA TOBACCO GUANGXI IND +1
External use emulsifiable paste for preventing and treating skin tinea
InactiveCN104997726AHigh antibacterial activityInhibition of growth and reproductionSalicyclic acid active ingredientsAntimycoticsSide effectTherapeutic effect
The invention belongs to the field of western medicines and relates to external use emulsifiable paste for preventing and treating skin tinea. The external use emulsifiable paste is prepared from 5-15% of terbinafine, 5-10% of chitosan, 3-7 % of Garenoxacin, 0.5-5% of sodium salicylate, 4-8% of chlorphenamine maleate, 5-15% of tretinoin, 3-7% of neomycin sulfate and the balance Vaseline. The external use emulsifiable paste has definite therapeutic effect of treating skin tinea, has high efficiency, reduces recurrence after cure, is safe and has toxic or side effect.
Owner:赵娟
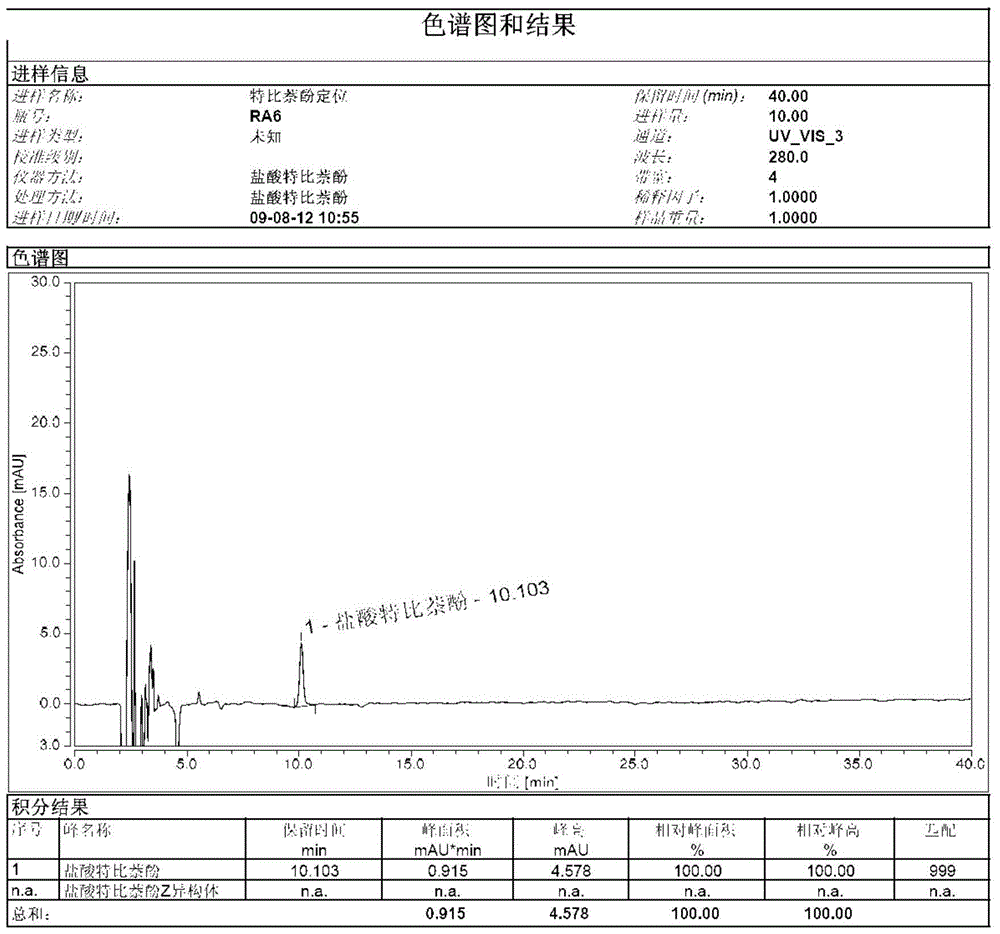
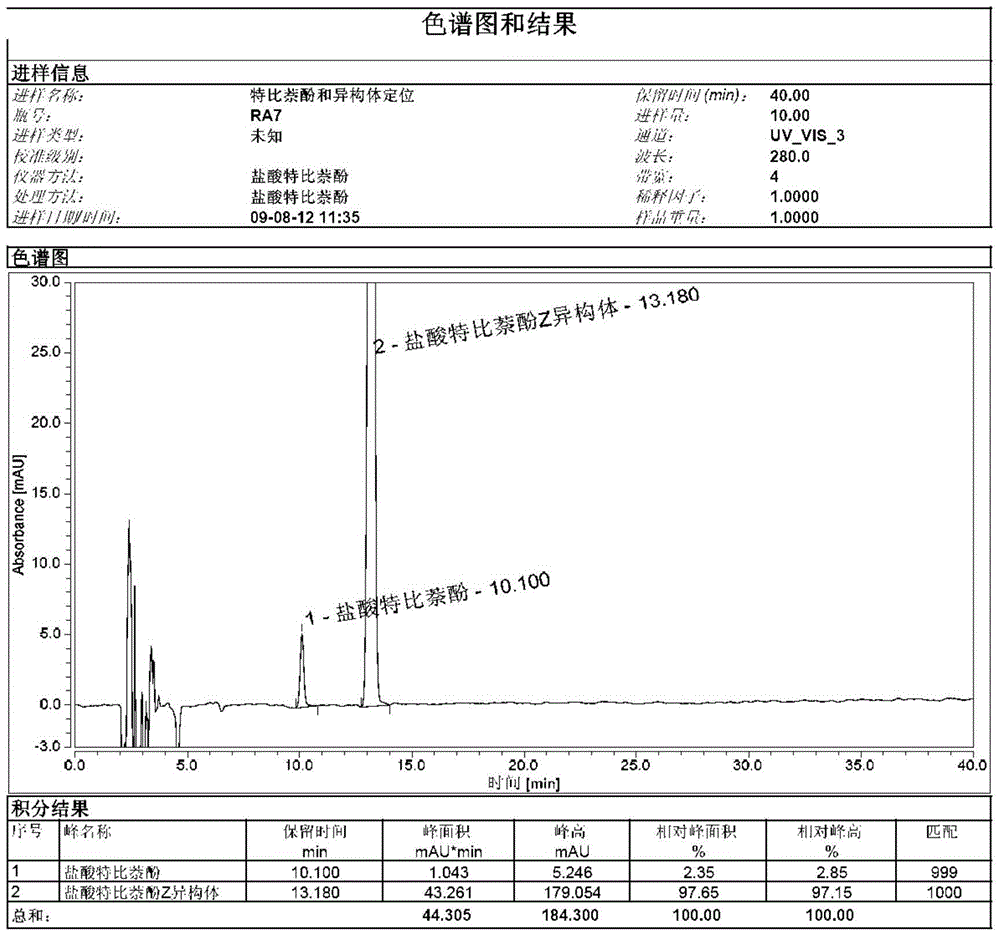
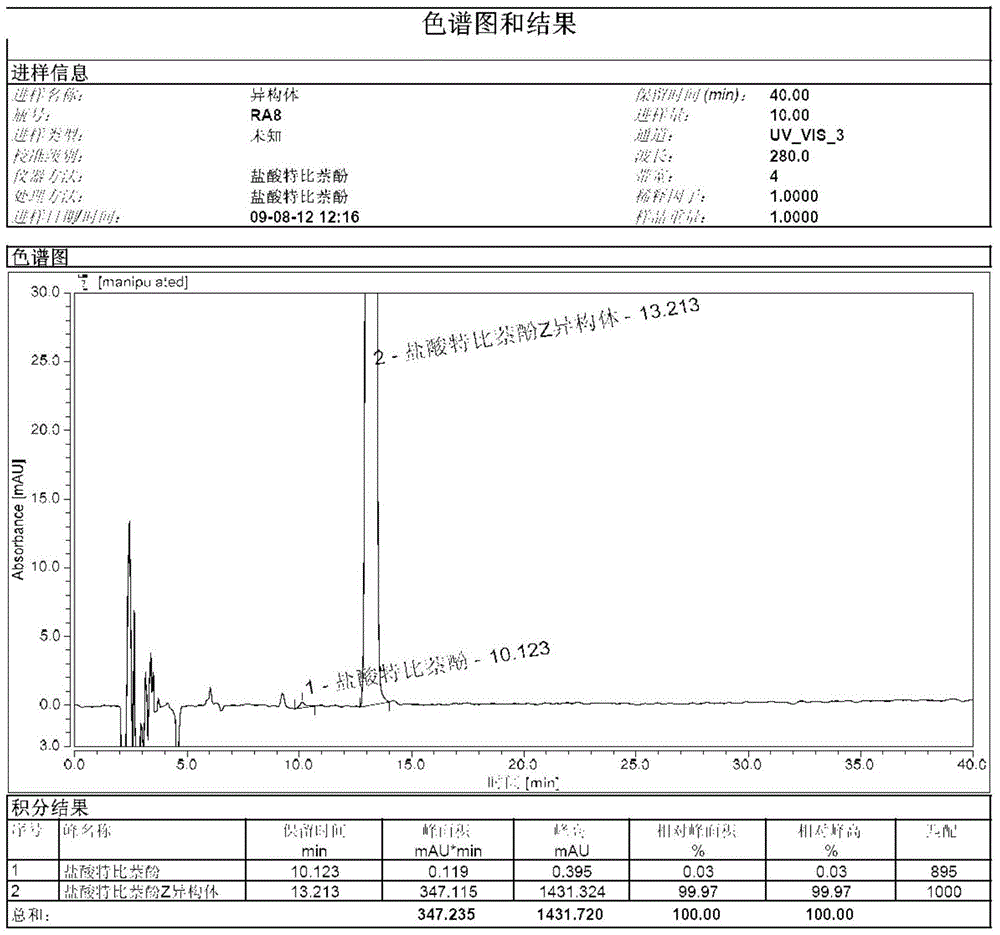
A kind of preparation method of terbinafine hydrochloride z type isomer
ActiveCN104725240BHigh purityAnalytical method is accurateAmino compound purification/separationEtherTert butyl
The invention discloses a method for preparing a terbinafine hydrochloride Z-shaped isomer serving as a high-purity isomer impurity compound for allylamine medicament terbinafine hydrochloride impurity analysis. The preparation method comprises the following steps: 1, adding a terbinafine EZ mixture to isopropanol, heating to 40-86 DEG C for dissolving, preserving the heat for 0.5-4 hours, slowly cooling to 10-20 DEG C, preserving the heat for 1-3 hours, cooling to -10-0 DEG C, preserving the heat for 1-5 hours, filtering, and concentrating the filtrate under reduced pressure to obtain an oily matter; and 2, adding 4-chlorphenyl-tert-butyl ether into the oily matter obtained in step 1, heating to 100-150 DEG C, preserving the heat for 1-5 hours, cooling to 0-40 DEG C, preserving the heat for 1-10 hours, and filtering to obtain an off-white compound. The method has the positive effect of obtaining the terbinafine hydrochloride isomer with relatively high purity. The isomer serving as a known impurity is used in quality analysis of terbinafine hydrochloride to determine the position of an impurity in a sample and survey the resolution between the impurity and the sample, so that the analysis method is more accurate. The preparation conditions are mild, the synthesis steps are simple, and the quality of the product is stable.
Owner:吉林修正药业新药开发有限公司
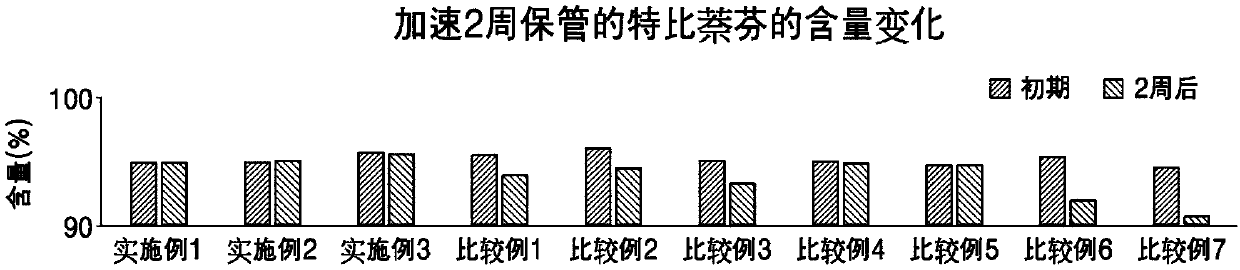
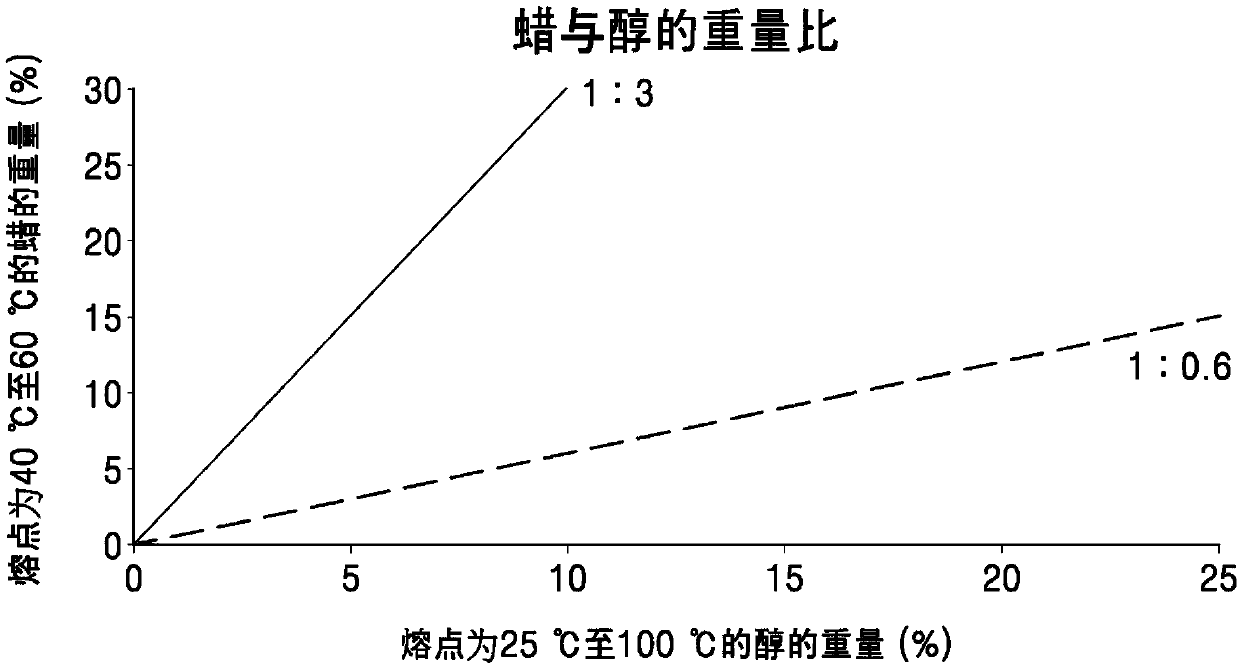
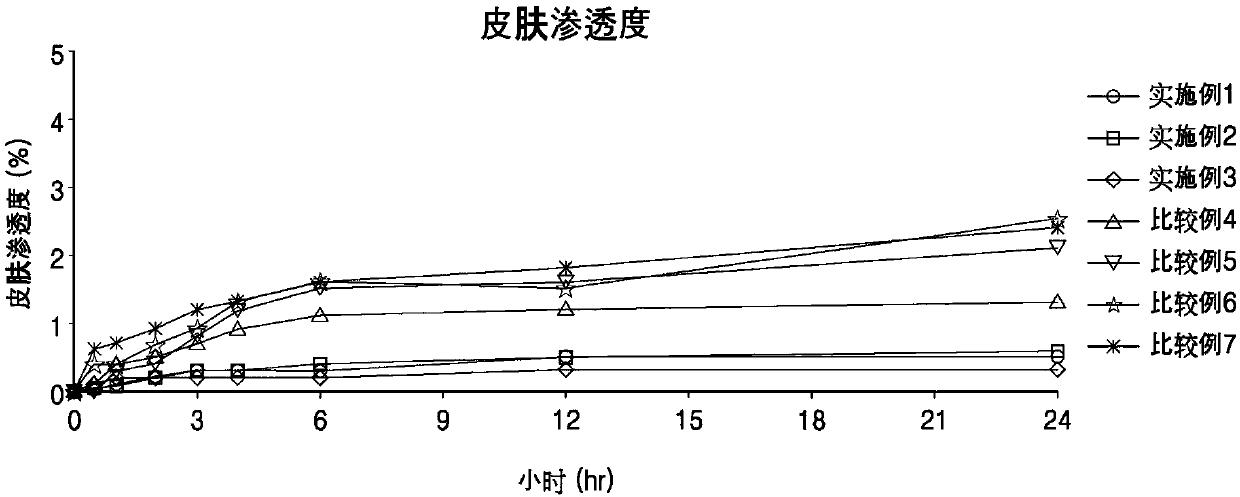
Topical composition comprising terbinafine with improved stability and preparation method thereof
The present invention provides an external preparation composition having improved preparation stability, drug therapy safety, and excellent patient convenience. The present invention relates to a composition for external use comprising terbinafine or a pharmaceutically acceptable salt thereof, an alcohol having a melting point of 25 DEG C to 100 DEG C and a wax having a melting point of 40 DEG Cto 60 DEG C. The present invention also provides a method for preparing the composition for external use.
Owner:HANMI PHARMA
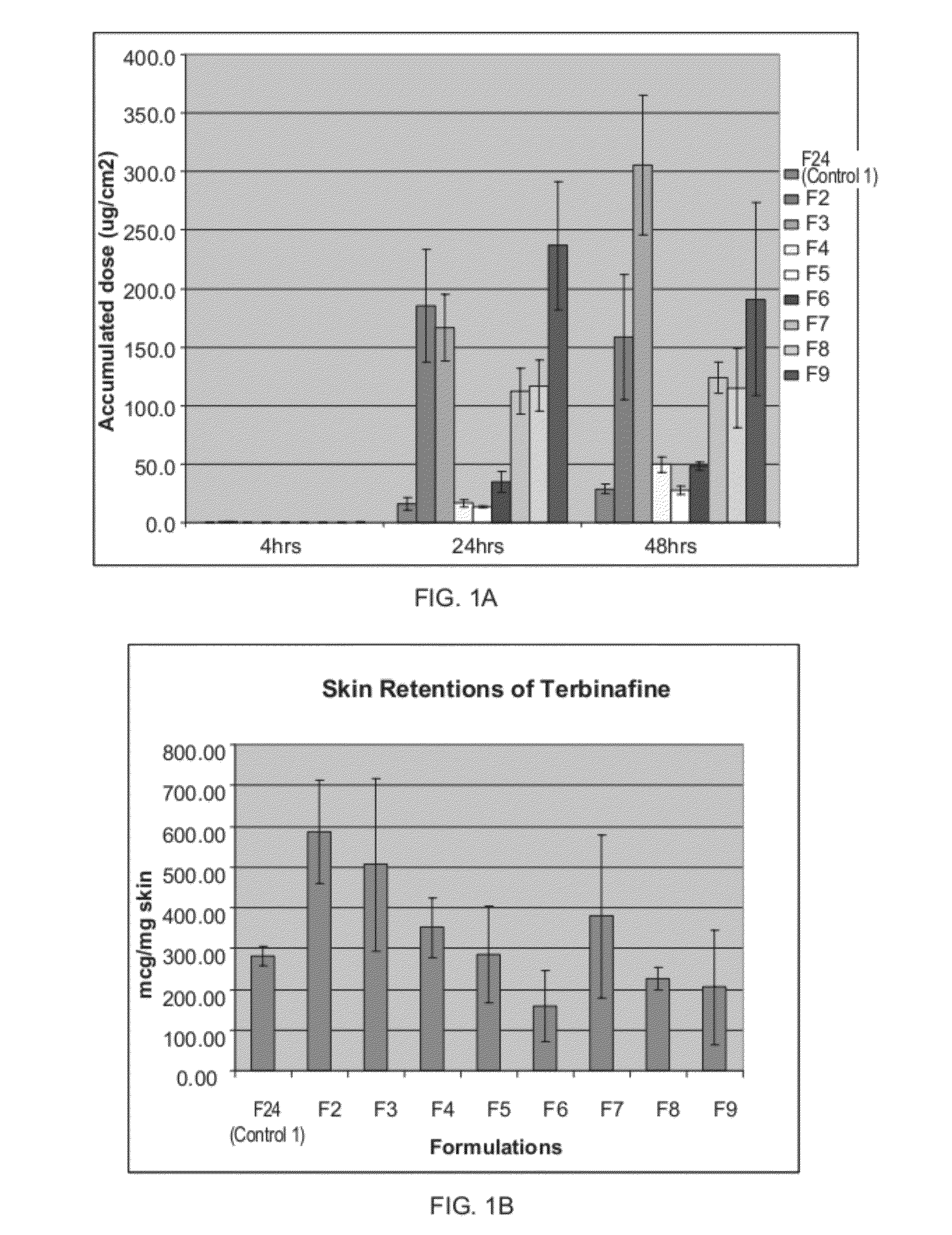
Highly permeating terbinafine formulation
Owner:CRESCITA THERAPEUTICS INC
Itraconazole-terbinafine compound injection for dogs and cats and preparation method of compound injection
InactiveCN105687196ABroad spectrum antibacterialGood curative effectOrganic active ingredientsAntimycoticsTherapeutic effectVeterinary Drugs
The invention relates to the field of veterinary drugs, and discloses an itraconazole-terbinafine compound injection for dogs and cats and a preparation method of the compound injection.The novel compound preparation prepared by taking hydroxypropyl-beta-cyclodextrin as a carrier has the advantages of being wide in antibacterial spectrum, high in treatment effect, low in cost and the like; the product is high in inclusion rate and drug concentration, capable of meeting the treatment requirement and good in stability; the compound injection is low in toxicity, good in absorption and capable of achieving the better treatment effect on fungal skin diseases of the dogs and the cats.
Owner:黑龙江省兽医科学研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com