Itraconazole-terbinafine compound injection for dogs and cats and preparation method of compound injection
The technology of itraconazole and injection is applied in the field of itraconazole-terbinafine compound injection for dogs and cats and its preparation field, which can solve the problems of fast acting time, short bioavailability, poor stability and the like , to achieve the effect of high drug concentration, high inclusion rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The invention discloses a method for preparing itraconazole-terbinafine compound injection for dogs and cats. The specific steps are as follows:
[0017] Step 1: Accurately weigh 1.0 g of itraconazole and 1.0 g of terbinafine into beaker A and beaker B respectively, divide hydroxypropyl-β-cyclodextrin into two parts, and put them into beaker C (containing hydroxyl Propyl-β-cyclodextrin 20~25g) and beaker D (containing hydroxypropyl-β-cyclodextrin 10~15g), add propylene glycol 5mL into beaker A, then slowly add 12mol·L -1 Concentrated hydrochloric acid 370μL to completely dissolve itraconazole; add 3mL of absolute ethanol to beaker B to completely dissolve terbinafine; pour double-distilled water into beaker C (containing 24-30mL of double-distilled water) and beaker D (containing Dissolve hydroxypropyl-β-cyclodextrin in 12-18 mL of double distilled water.
[0018] Step 2: Under the condition of magnetic stirring, slowly drop the itraconazole solution in beaker A into b...
Embodiment 2
[0022] Experimental purpose and method: In order to characterize the chemical relationship between hydroxypropyl-β-cyclodextrin, itraconazole and terbinafine, this example uses ultraviolet absorption spectrum to characterize and analyze the synthetic preparation. The experimental operation is consistent with the relevant standard operation, and will not be repeated here.
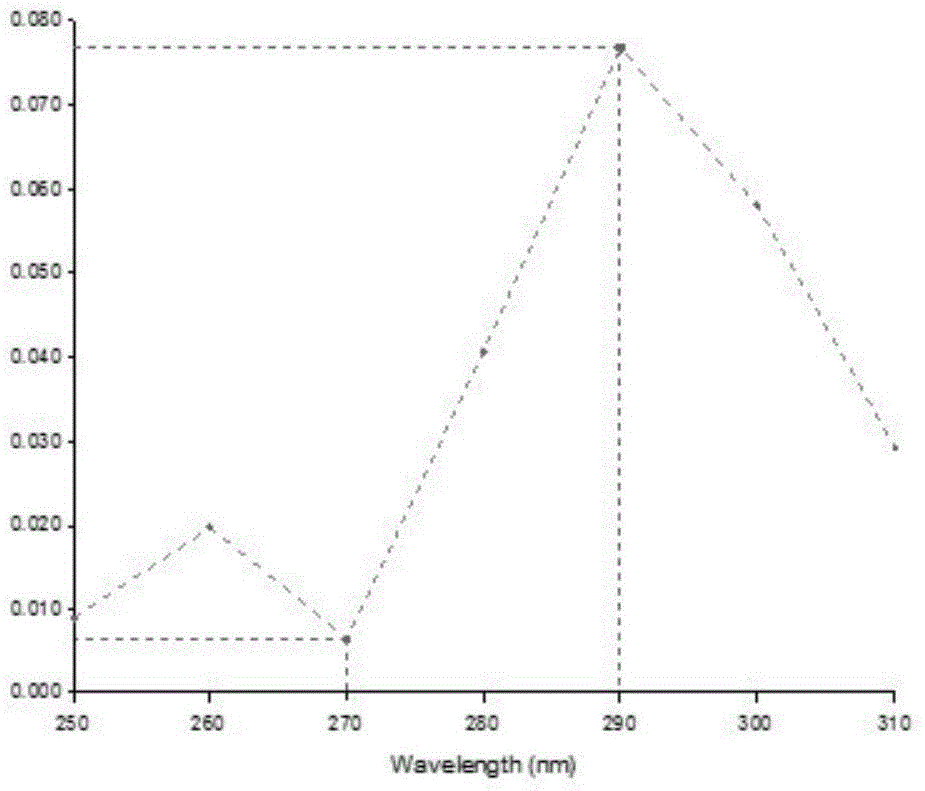
[0023] Experimental results: if figure 1 As shown, use a microplate reader to carry out wavelength scanning of the compound injection and perform absorption band scanning in the range of 250nm to 310nm (λ), by figure 1 It can be seen that the drug in the compound injection forms peaks at 262nm and 289nm, indicating that itraconazole and terbinafine did not react, but formed inclusion complexes with hydroxypropyl-β-cyclodextrin respectively.
Embodiment 3
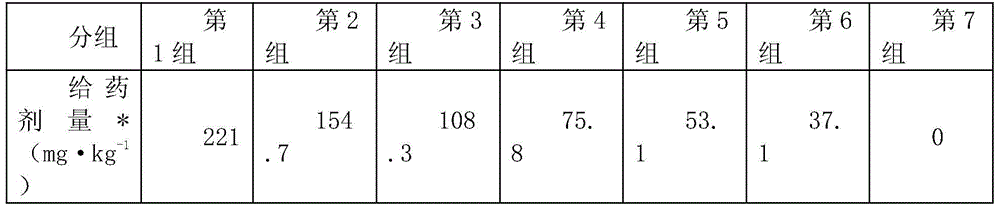
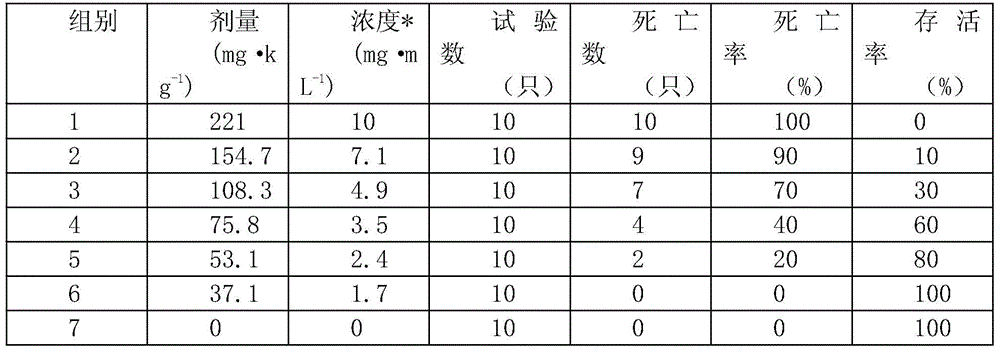
[0025] Experimental purpose and method: In order to characterize the biological toxicity of the preparation of the present application, this example established a mouse model, and carried out an acute toxicity test and a subchronic toxicity test. The specific experimental methods are as follows: Acute toxicity test: after pre-experimentation, compound injection The median lethal dose (LD50) of terbinafine in solution is higher than that of itraconazole, so the dosage is calculated by itraconazole. 70 mice were randomly divided into 7 groups, 10 in each group, half male and half male, and kept in separate cages. Among them, the seventh group is the control group (injection of normal saline). The doses of the mice in each group were determined on the basis of the pre-test, and the dose ratio between two adjacent groups was 1.4. The specific doses of the mice in each group are shown in Table 1. Press 0.03mL·g for each mouse -1 Subcutaneous injection on the back of the body weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com