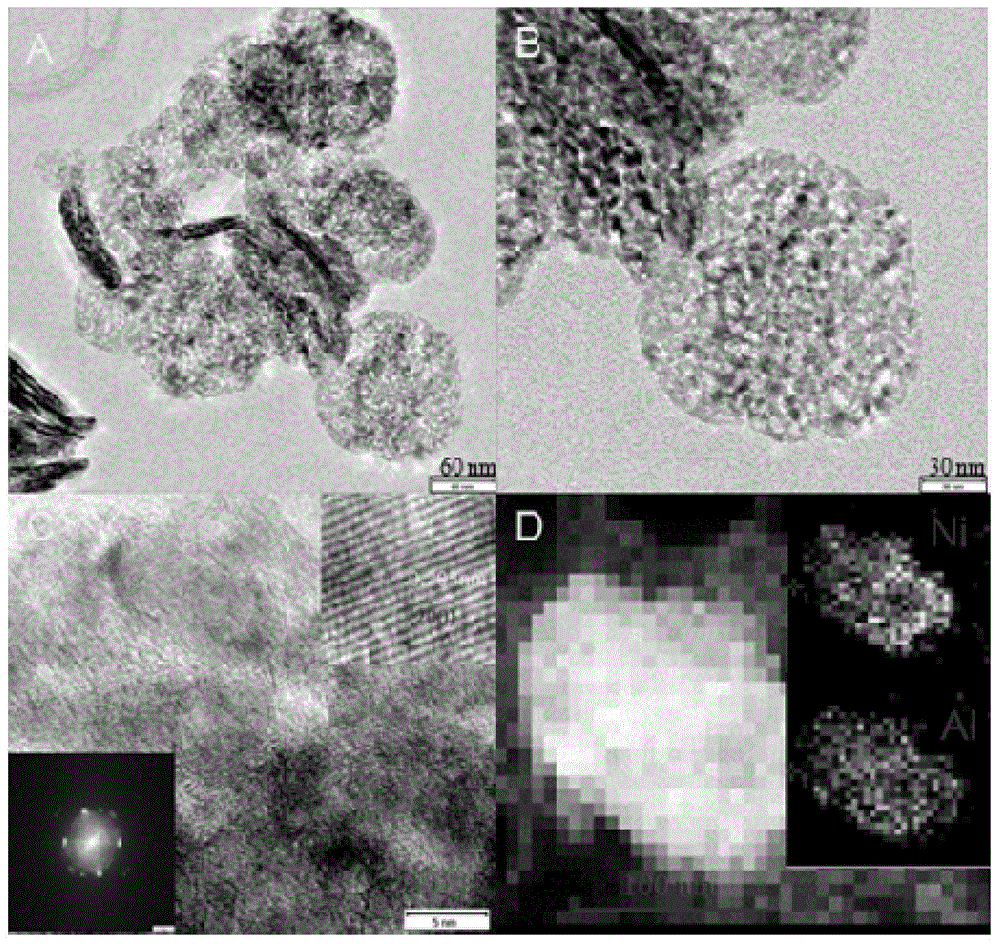
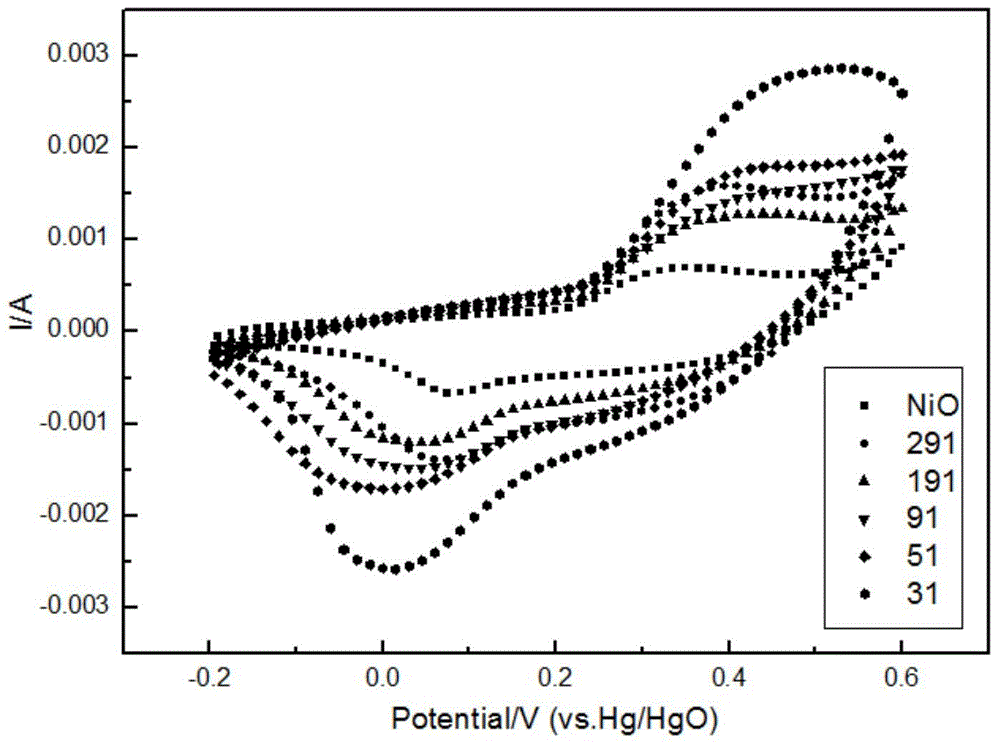
Method for preparing aluminum-doped nickel oxide electrochromic film by virtue of hydrotalcite precursor pyrolysis
An electrochromic and aluminum oxidation technology, which is applied in the field of preparing aluminum-doped nickel oxide electrochromic films by pyrolysis of hydrotalcite precursors, can solve the problems of poor cycle stability and slow electrochromic speed, and achieves fast transformation speed, good Effects of Cyclic Stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Prepare hydrotalcite by co-precipitation method, the specific method is to weigh 11.632g Ni(NO 3 ) 2 ·6H 2 O and 5gAl(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 100mL mixed salt solution, and 4.68g NaOH and 3.77g NaOH were weighed 2 CO 3 Dissolve in deionized water to make 100mL alkali solution, drop the two mixed solutions into a three-necked flask filled with 120ml deionized water at the same time and with mechanical stirring, in a water bath at 40°C, adjust the reaction to a final pH of 10, and crystallize for 24 hours. Centrifugal washing, drying, and grinding into powder, the chemical formula of the obtained nickel aluminum hydrotalcite is: Ni 0.753 Al 0.247 (OH) 2 (CO 3 ) 0.124 ·H 2 O;
[0023] 2. Weigh 10 mg of the nickel-aluminum hydrotalcite powder prepared in step 1 and disperse it in 10 ml of ethanol, and ultrasonicate for 20 minutes to obtain a colloidal solution; select a 2 cm*3 cm ITO sheet, and soak it in dilute hydrochloric a...
Embodiment 2
[0029] 1. Prepare hydrotalcite by co-precipitation method, the specific method is to weigh 11.632g Ni(NO 3 ) 2 ·6H 2 O and 3.75gAl(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 100mL mixed salt solution, and 4.4g NaOH and 3.551g NaOH were weighed 2 CO 3 Dissolve in deionized water to make 100mL alkali solution, drop the two mixed solutions into a three-necked flask filled with 120ml deionized water at the same time and with mechanical stirring, in a 40°C water bath, adjust the reaction to a final pH of 10, centrifuge, wash, and dry , ground into powder, the chemical formula of the obtained nickel aluminum hydrotalcite is: Ni 0.831 Al 0.169 (OH) 2 (CO 3 ) 0.085 0.8H 2 O;
[0030] 2. With embodiment 1;
[0031] 3. With embodiment 1;
[0032] 4. With embodiment 1;
[0033] 5. Same as embodiment 1.
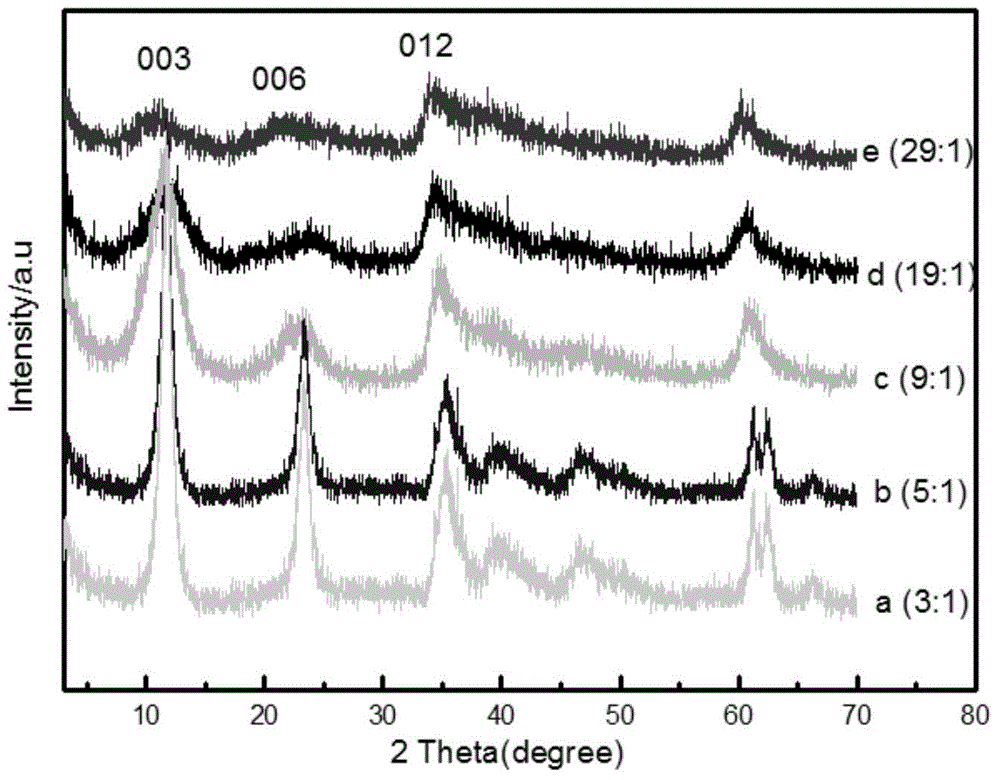
[0034] To characterize the product: by figure 1 The characteristic peaks such as 003, 006, and 012 in b can be known as the XRD pattern of nickel aluminum ...
Embodiment 3
[0036] 1. Prepare hydrotalcite by co-precipitation method, the specific method is to weigh 11.632g Ni(NO 3 ) 2 ·6H 2 O and 1.67gAl(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 100mL mixed salt solution, and 3.91g NaOH and 3.16g NaOH were weighed 2 CO 3 Dissolve in deionized water to make 100mL alkali solution, drop the two mixed solutions into a three-necked flask filled with 120ml deionized water at the same time and with mechanical stirring, in a 40°C water bath, adjust the reaction to a final pH of 10, centrifuge, wash, and dry , ground into powder, the chemical formula of the obtained nickel aluminum hydrotalcite is: Ni 0.895 al 0.105 (OH) 2 (CO 3 ) 0.052 2H 2 O;
[0037] 2. With embodiment 1;
[0038] 3. With embodiment 1;
[0039] 4. With embodiment 1;
[0040] 5. Same as embodiment 1.
[0041] To characterize the product: by figure 1 The characteristic peaks such as 003, 006, and 012 in c can be known as the XRD pattern of nickel aluminum ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com