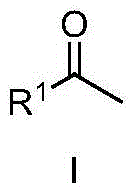
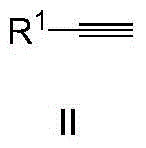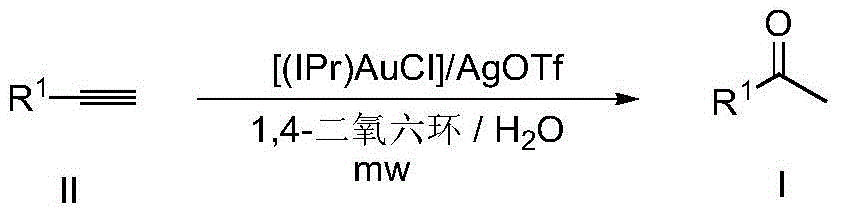Method for synthesizing methyl ketone
A technology of methyl ketone and synthesis reaction, which is applied in hydrolysis to prepare carbonyl compounds, carbon-carbon triple bond hydration preparation, organic chemistry, etc. It can solve long-term problems, achieve broad development prospects, and shorten the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: acetophenone
[0021] Acetophenone
[0022]
[0023] Catalyst A (6mg, 0.01mmol, 1.0mol%), AgOTf (2.6mg, 0.01mmol, 1.0mol%), phenylacetylene (102mg, 1mmol), 1,4-dioxane (1ml) and water (36ul ) were sequentially added to 5ml microwave tubes. The reaction mixture was microwaved at 120 °C for 1 hour and then cooled to room temperature. Filtrate, remove the solvent by rotary evaporation, and then obtain the pure target compound by column chromatography (developing solvent: petroleum ether / ethyl acetate), yield: 92%
[0024] 1 H NMR (500MHz, CDCl 3 )δ7.96(dd,J=7.6Hz and0.7Hz,2H,ArH),7.57(t,J=7.5Hz,1H,ArH),7.47(t,J=7.7Hz,2H,ArH),2.61( s,3H,CH 3 ).
Embodiment 2
[0025] Embodiment 2:4-methylacetophenone
[0026] 1-p-tolylethanone
[0027]
[0028] Catalyst A (6mg, 0.01mmol, 1.0mol%), AgOTf (2.6mg, 0.01mmol, 1.0mol%), 4-methylphenylacetylene (116mg, 1mmol), 1,4-dioxane (1ml) and water (36ul) were sequentially added to a 5ml microwave tube. The reaction mixture was microwaved at 120 °C for 1 hour and then cooled to room temperature. Filtrate, remove the solvent by rotary evaporation, and then obtain the pure target compound by column chromatography (developing solvent: petroleum ether / ethyl acetate), yield: 93%
[0029] 1 H NMR (500MHz, CDCl 3 )δ7.86(d,J=7.9Hz,2H,ArH),7.27(d,J=6.4Hz,2H,ArH),2.59(s,3H,CH 3 ),2.42(s,3H,CH 3 ).
Embodiment 3
[0030] Embodiment 3:4-methoxyacetophenone
[0031] 1-(4-methoxyphenyl)ethanone
[0032]
[0033] Catalyst A (6mg, 0.01mmol, 1.0mol%), AgOTf (2.6mg, 0.01mmol, 1.0mol%), 4-methoxyphenylacetylene (132mg, 1mmol), 1,4-dioxane (1ml ) and water (36ul) were sequentially added to a 5ml microwave tube. The reaction mixture was microwaved at 120 °C for 1 hour and then cooled to room temperature. Filtration, rotary evaporation to remove the solvent, and then column chromatography (developing solvent: petroleum ether / ethyl acetate) to obtain the pure target compound, yield: 94%
[0034] 1 H NMR (500MHz, CDCl 3 )δ7.94(d,J=8.8Hz,2H,ArH),6.94(d,J=8.8Hz,2H,ArH),3.88(s,3H,OCH 3 ),2.57(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com