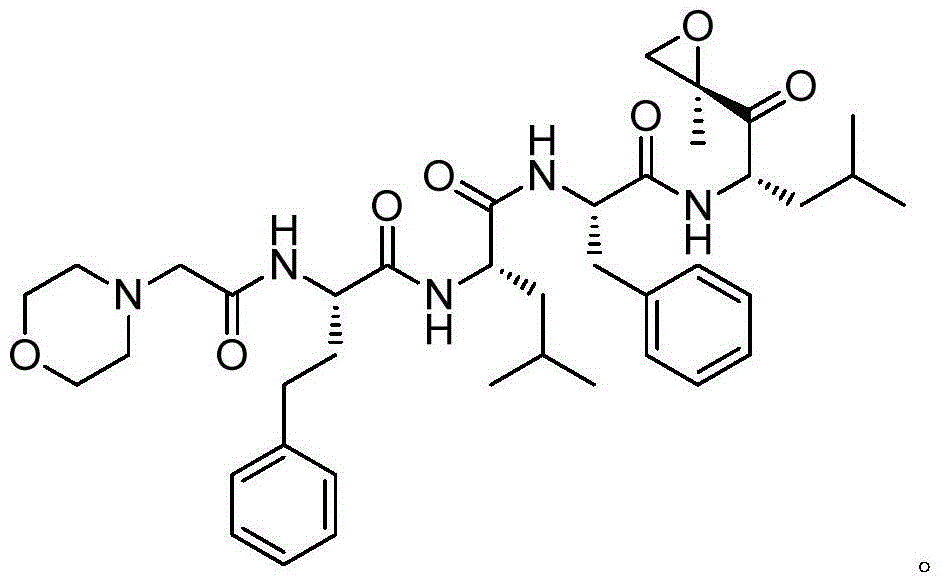
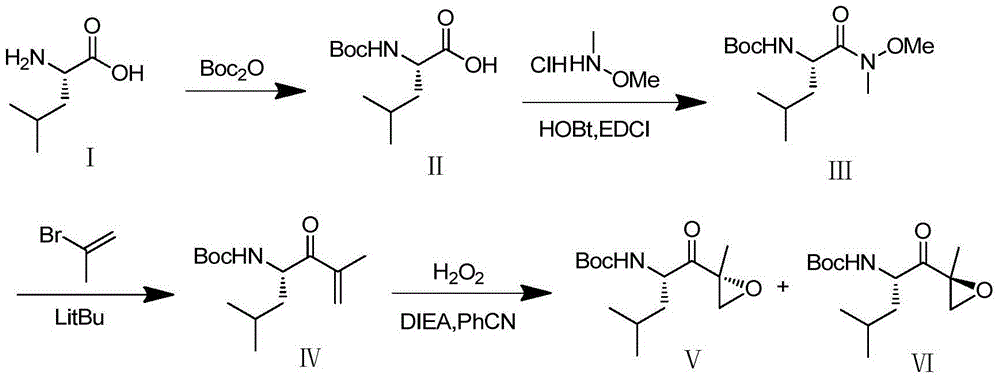
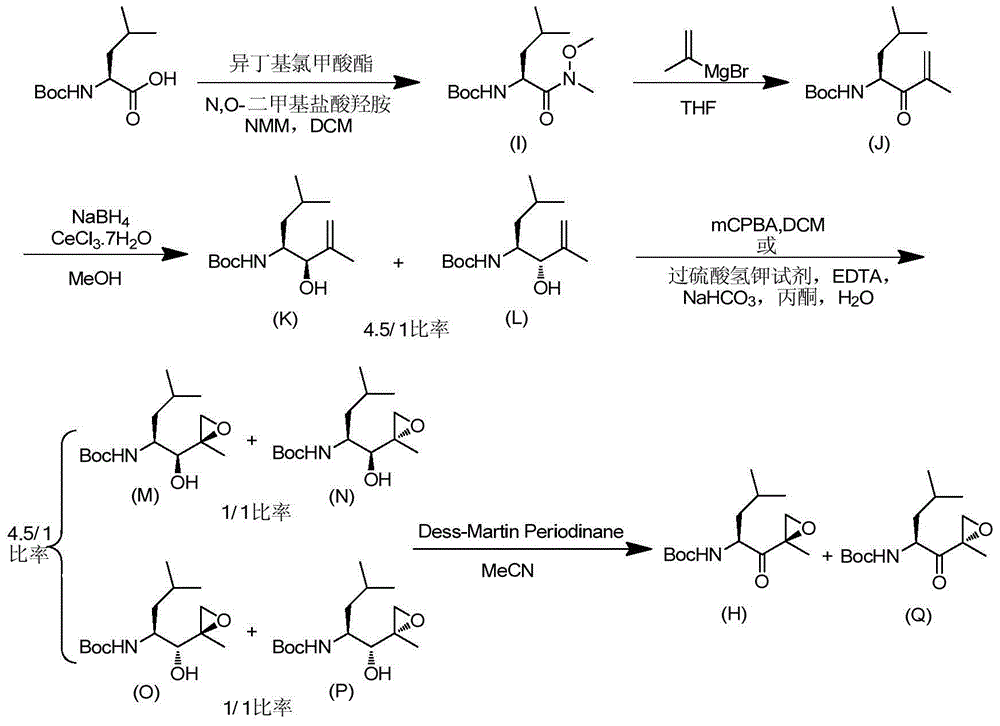
Synthetic method of carfilzomib intermediate and carfilzomib intermediate
A synthesis method and carfilzomib technology are applied in chemical instruments and methods, preparation of organic compounds, preparation of carbamate derivatives, etc., and can solve the problems of long reaction route, complicated process, low overall yield, etc. The effect of high production cost, high reaction yield and concise process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of compound 5a
[0064]
[0065] Dissolve 231g (1.0mol, 1eq) of 6a in 2L of dichloromethane, add 195g (1.2mol, 1.2eq) of carbonyldiimidazole in batches within half an hour at room temperature, stir at room temperature for 1 hour, add 138g (3.0 mol, 3.0eq) ethanol, continued to stir at room temperature for 4 hours, sampling TLC showed that the reaction was complete. Add 500ml of water, stir and separate the layers, and the organic phase is successively washed with 500ml of saturated sodium bicarbonate, 500ml of 1N aqueous hydrochloric acid, 500ml of water, and 200ml of saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 246.4g of a light yellow oily liquid. Yield 95.0%, HPLC purity 99.9%.
Embodiment 2
[0067] Synthesis of compound 5b
[0068]
[0069] Dissolve 265.3g (1.0mol, 1eq) of 6b in 2L of dichloromethane, add 195g (1.2mol, 1.2eq) of carbonyldiimidazole in batches within half an hour at room temperature, after the addition is complete, stir at room temperature for 1 hour, then add 138g ( 3.0mol, 3.0eq) ethanol, continued to stir at room temperature for 4 hours, sampling TLC showed that the reaction was complete. Add 500ml of water, stir and separate the layers, and the organic phase is washed successively with 500ml of saturated sodium bicarbonate, 500ml of 1N aqueous hydrochloric acid, 500ml of water, and 200ml of saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 287.3g of light buttery liquid. Yield 98.0%, HPLC purity 99.7%.
Embodiment 3
[0071] Synthesis of compound 5c
[0072]
[0073] Dissolve 231g (1.0mol, 1eq) of 6a in 2L of dichloromethane, add 195g (1.2mol, 1.2eq) of carbonyldiimidazole in batches within half an hour at room temperature, after the addition is complete, stir at room temperature for 1 hour, then add 324.4g ( 3.0mol, 3.0eq) benzyl alcohol, continued to stir at room temperature for 4 hours, sampling TLC showed that the reaction was complete. Add 500ml of water, stir and separate the layers, and the organic phase is successively washed with 500ml of saturated sodium bicarbonate, 500ml of 1N aqueous hydrochloric acid, 500ml of water, and 200ml of saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 311.8g of light buttery liquid. Yield 97.0%, HPLC purity 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com