Rabies virus attenuated strains as well as breeding method and application thereof
A technology of rabies virus and attenuated strains, applied in the field of virus vaccines, to achieve good and stable immunogenicity, low residual virulence, and clear background of virus species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, adaptive passage and plaque purification of rabies virus CTN-181 and CTN-181-SG strains
[0025] 1. Adaptation of rabies-fixed toxic human diploid cells to subculture and plaque purification
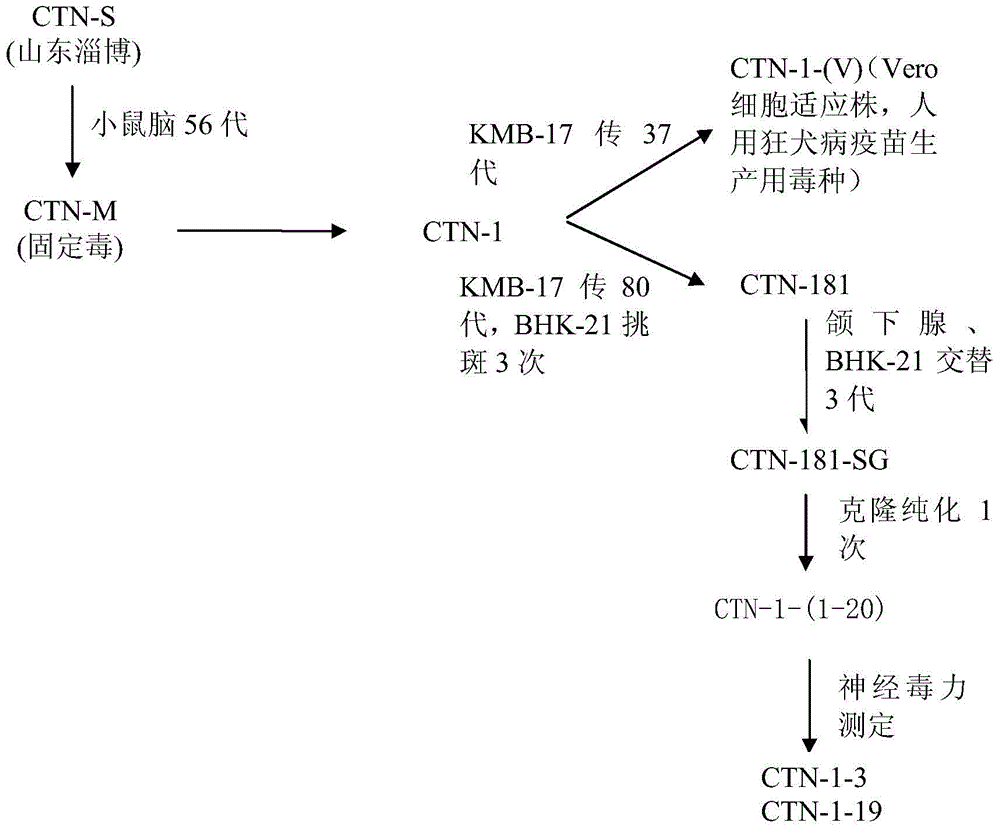
[0026] Rabies virus CTN strain was isolated from the brain tissue of rabies patients in Zibo, Shandong Province by the applicant, and the fixed strain CTN-M strain was obtained by passage in the brain of mice for 56 generations. Passage to the 80th generation, the virus seeds of the 80th generation were purified three times according to the neutral red plaque to obtain the CTN-181 strain.
[0027] 2. Submandibular gland passage of guinea pigs
[0028] Take the CTN-181 strain and inject several guinea pigs subcutaneously in the neck with the stock solution, each inoculate 1ml, take 2 guinea pigs on the second day after inoculation, and use CO 2 Put it to death, soak it in 3% Lysol water for 5 minutes, take out the guinea pig and put it on its back on double-layer ga...
Embodiment 2
[0031] Example 2, Screening of low residual neurovirulence clones
[0032] 1. Method
[0033] 1. Neurovirulence screening in the brain of 3-week-old mice
[0034] The CTN-1-(1-20) prepared above was quickly melted under running water, and the stock solution (10 0 ) do 10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 6 10-fold serial dilutions, take 10 0 -10 -3 Four groups of dilute doses were inoculated into the brain of 6 3-week-old Kunming mice, 0.03ml / only, and another 6 mice were inoculated with PBS in the brain as a blank control. On the fourth day, observe whether there was non-specific death. Days, the incidence and death of mice were recorded, and the LD of the clone was calculated as the cumulative mortality of four dilutions 50 . take 10 -4 -10 -6 The diluted virus solution and the blank dilution solution were used for simultaneous plaque titration, and the PFU value was calculated.
[0035]2. Screening of neurotoxicity in the brain of 5-day-old, 10-day-old, an...
Embodiment 3
[0051] Embodiment 3, amplification, titration and determination of CTN-1-3, CTN-1-19 attenuated strain
[0052] 1. Amplification of virus species
[0053] After culturing BHK-21 cells into a dense monolayer, the virus seeds were inoculated into CTN-1-3 and CTN-1-19 strains at an infection rate of 0.1 MOI, and the cells were incubated at 37°C in 5% CO 2 Discard the culture medium after adsorption for 1 hour, rinse with sterilized PBS, add an appropriate amount of maintenance solution, transfer to 35°C to continue the culture, and collect the culture supernatant when the cell lesion reaches "+++", and centrifuge at 4000r / min for 10 minutes at 4°C. Remove cell debris within 1 minute, take the supernatant and add 10% calf serum, mix well, divide into small tubes, and freeze at -70°C for later use.
[0054] 2. Virus species titration
[0055] Take one of each of the frozen virus species, and make six 10-fold serial dilutions starting from 1:10 on the 24-well culture plate (10 -1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com