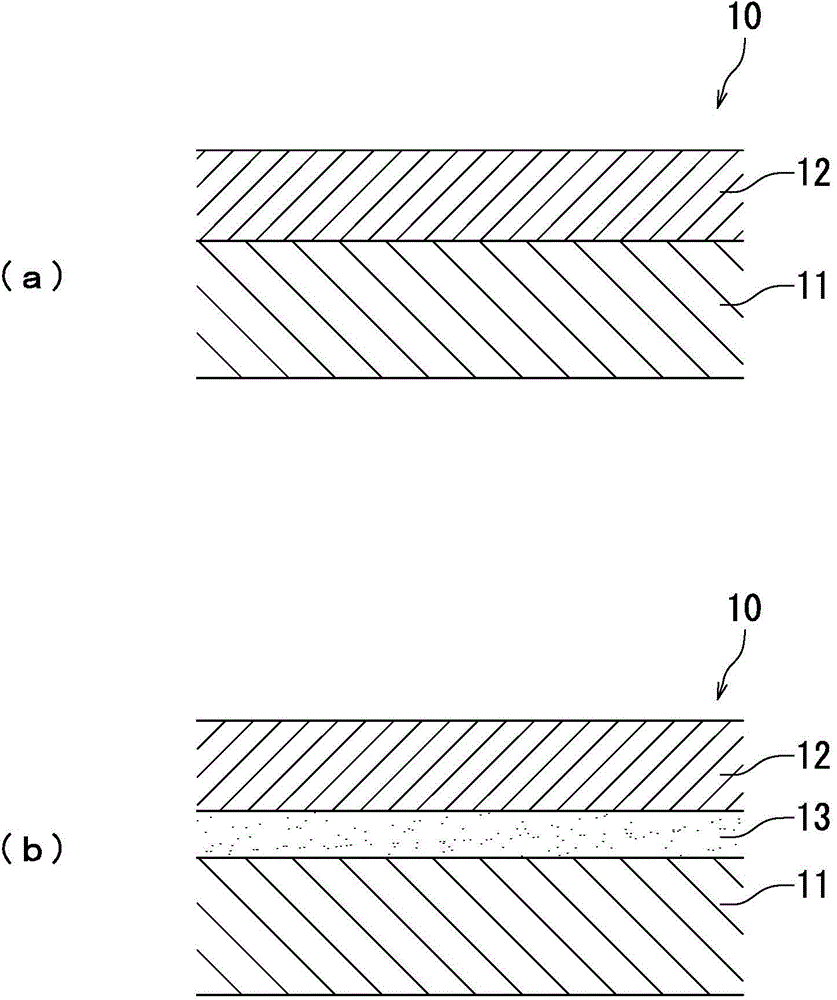
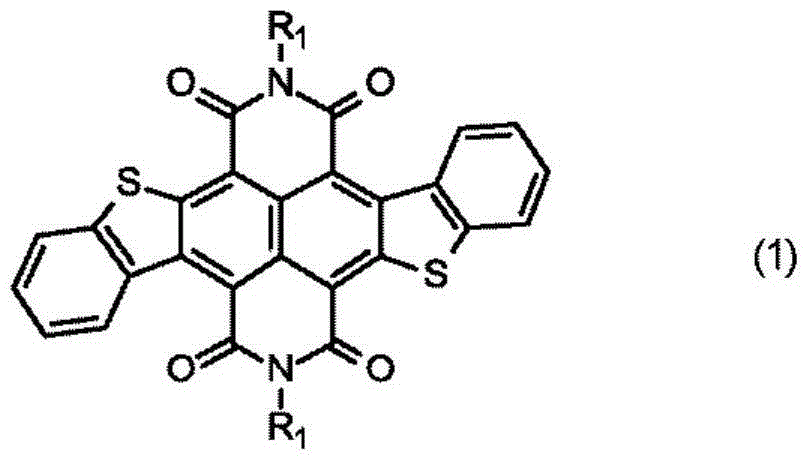
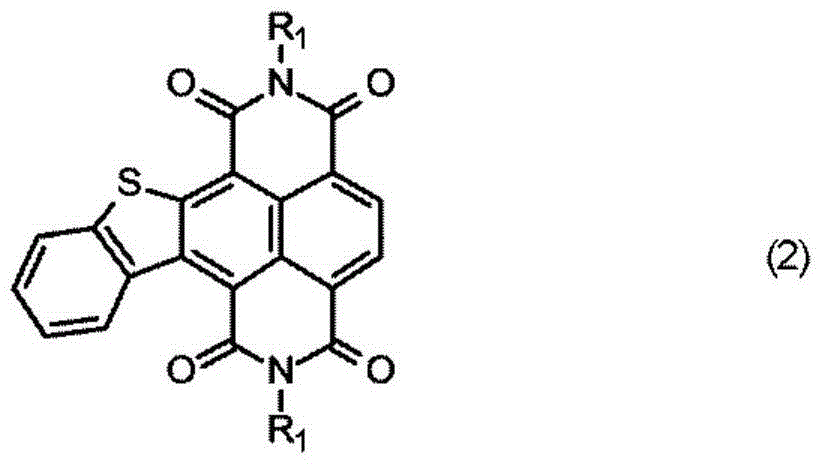
Electrophotographic photosensitive member
An electrophotographic and photoreceptor technology, applied in optics, electrographics, instruments, etc., can solve problems such as difficulty in exerting sensitivity characteristics, and achieve the effect of excellent sensitivity characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0080] The naphthalene diimide derivative represented by the following general formula (1-2) was synthesize|combined according to the following reaction scheme 3.
[0081] [Electron Transport Agent (ETM-1)]
[0082] [chemical formula 8]
[0083]
[0084] [Reaction Equation 3]
[0085] [chemical formula 9]
[0086]
[0087] In reaction equation 3 (a-2), compound (A-12) 0.875g (1mmol), 2-bromoanisole 0.812g (4mmol), tetrakis (triphenylphosphine) palladium 58mg (0.05mmol) and iodine A toluene solution of 19 mg (0.1 mmol) of cuprous chloride was stirred under reflux for 10 hours under a nitrogen atmosphere at a temperature of 110° C. to obtain a reaction solution.
[0088] The toluene solvent in the obtained reaction solution was distilled off and purified by column chromatography to obtain 0.6 g of compound (A-22) (yield: 85%).
[0089] In the reaction equation 3 (b-2), the solution of compound (A-22) 0.708g (1mmol), acetic acid 10ml and chloroform 10ml is cooled with i...
Synthetic example 2
[0094] The naphthalene diimide derivative represented by the following general formula (1-3) was synthesize|combined according to the following reaction scheme 4.
[0095] [Electron Transport Agent (ETM-2)]
[0096] [chemical formula 10]
[0097]
[0098] [Reaction Equation 4]
[0099] [chemical formula 11]
[0100]
[0101] In reaction equation 4 (a-3), compound (A-13) 0.78g (1mmol), 2-bromoanisole 0.81g (4mmol), tetrakis (triphenylphosphine) palladium 58mg (0.05mmol) and iodine A toluene solution of 19 mg (0.1 mmol) of cuprous chloride was stirred under reflux for 10 hours under a nitrogen atmosphere at a temperature of 110° C. to obtain a reaction solution.
[0102] The toluene solvent in the obtained reaction solution was distilled off and purified by column chromatography to obtain 0.49 g of compound (A-23) (yield: 80%).
[0103] Next, in reaction equation 4 (b-3), the solution of compound (A-23) 0.61g (1mmol), acetic acid 10ml and chloroform 10ml was cooled wit...
Synthetic example 3
[0107] The naphthalene diimide derivative represented by the following general formula (1-4) was synthesize|combined according to the following reaction scheme 5.
[0108] [Electron Transport Agent (ETM-3)]
[0109] [chemical formula 12]
[0110]
[0111] [Reaction Equation 5]
[0112] [chemical formula 13]
[0113]
[0114] In reaction equation 5 (a-4), compound (A-14) 1.17g (1mmol), 2-bromoanisole 1.62g (4mmol), tetrakis (triphenylphosphine) palladium 58mg (0.05mmol) and iodine A toluene solution of 19 mg (0.1 mmol) of cuprous chloride was stirred under reflux for 10 hours under a nitrogen atmosphere at a temperature of 110° C. to obtain a reaction solution.
[0115] The toluene solvent in the obtained reaction solution was distilled off, followed by purification by column chromatography to obtain 0.66 g of compound (A-24) (yield: 80%).
[0116] Then, in the reaction equation 5 (b-4), the solution of compound (A-24) 0.83g, acetic acid 10ml and chloroform 10ml was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com