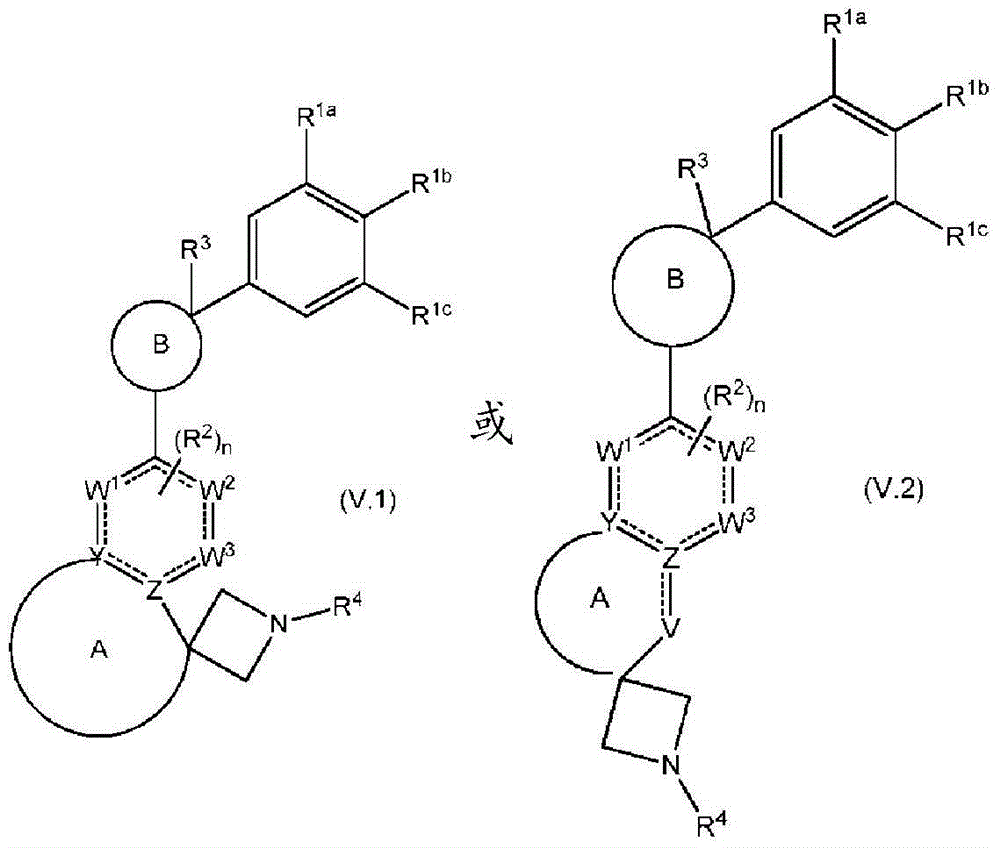
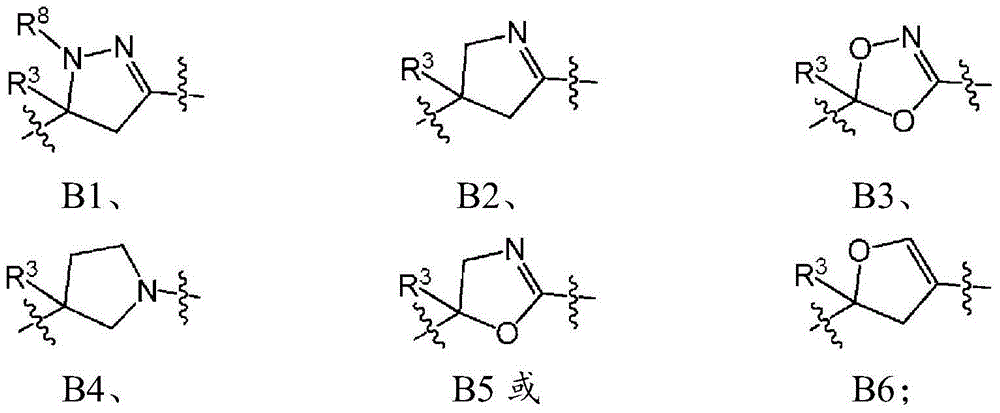
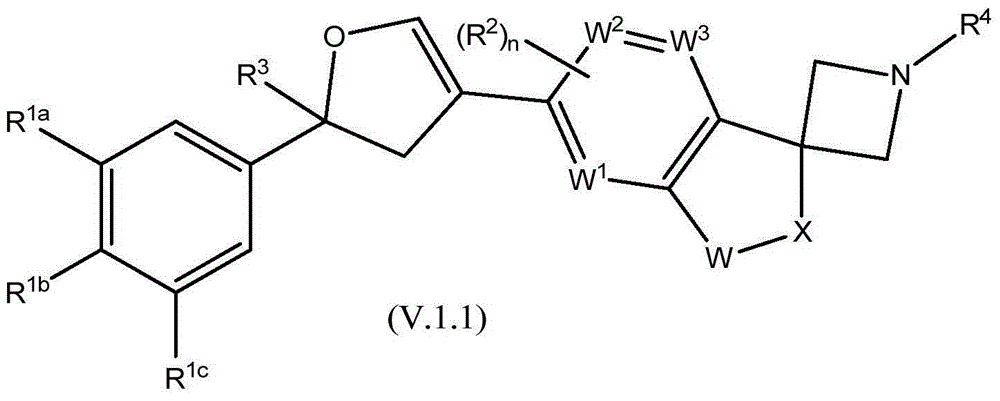
Spirocyclic derivatives as antiparasitic agents
A compound, cycloalkyl technology, applied in the field of manufacturing said spiro derivatives, can solve the problem of not indicating the range of life cycle stages of parasite species, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0191] In the preparation of compounds of the present invention, protection of remote functional groups of intermediates from undesired reactions can be accomplished with protecting groups. The term "protecting group" or "Pg" refers to a substituent typically used to block or protect a particular functional group while allowing other functional groups on the compound to react. For example, an amine protecting group is an amine-attached substituent that blocks or protects the amine functionality of a compound or intermediate. Suitable amine protecting groups include: 1-tert-butoxycarbonyl (Boc); acyl groups including: formyl, acetyl, chloroacetyl, trichloro-acetyl, o-nitrophenylacetyl, o-nitrophenoxy Acetyl, trifluoroacetyl, acetoacetyl, 4-chlorobutyryl, isobutyryl, o-nitrocinnamoyl, picolinoyl, acyl isothiocyanate, aminocaproyl, benzoyl, etc.; and acyloxy, including: methoxycarbonyl, 9-fluorenyl-methoxycarbonyl, 2,2,2-trifluoroethoxycarbonyl, 2-trimethylsilylethoxycarbonyl, v...
example
[0256] The following examples provide a more detailed description of the process conditions for preparing compounds of the invention. It should be understood, however, that the invention, as fully described herein and as recited in the claims, is not intended to be limited to the details of the following schemes or preparations. Applicable (E / Z) nomenclature for each of the preparation intermediates is also contemplated.
[0257] Intermediate 1 : tert-butyl 5'-bromo-3'H-spiro[azetidine-3,1'-isobenzofuran]-1-carboxylate
[0258]
[0259] Compound 4-bromo-2-(chloromethyl)-1-iodobenzene (500 g, 1.509 mol) was dissolved in tetrahydrofuran (3750 mL) and cooled to -20°C. i-PrMgCl-LiCl (1.3M solution in THF) (1275 mL, 1.66 mol) was added at less than -15°C. The reaction mixture was cooled to -20°C. tert-butyl 3-oxo-azetidine-1-carboxylate (310 g, 1.81 mol) was added as a solution in tetrahydrofuran (750 mL). The reaction was allowed to warm to room temperature over 90 minute...
example 1
[0275] Example 1: 2-(methylsulfonyl)-1-(5'-(3-(3,4,5-trichlorophenyl)-3-(trifluoromethyl)-3,4-dihydro-2H -pyrrol-5-yl)-3'H-spiro[azetidine-3,1'-isobenzofuran]-1-yl)ethanone
[0276]
[0277] To 5'-(3-(3,4,5-trichlorophenyl)-3-(trifluoromethyl)-3,4-dihydro-2H-pyrrol-5-yl)-3' at room temperature To a stirred solution of H-spiro[azetidine-3,1'-isobenzofuran] hydrochloride (Preparation 5, 900 mg, 1.77 mmol) in THF (20 mL) was added DIPEA (3.01 mL, 17.65 mmol ), methanesulfonylacetic acid (488mg, 3.53mmol), then add T 3 P (5.12 mL, 8.82 mmol, 50% in ethyl acetate). The resulting reaction mixture was stirred at room temperature for 16 hours. The reaction was monitored by TLC, and after complete consumption of the starting material, the reaction mixture was quenched with water (30 mL) and extracted with ethyl acetate (3 x 25 mL). The combined organic layers were washed with NaHCO 3 The solution (30 mL) was washed with brine (30 mL), dried over sodium sulfate, and concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com