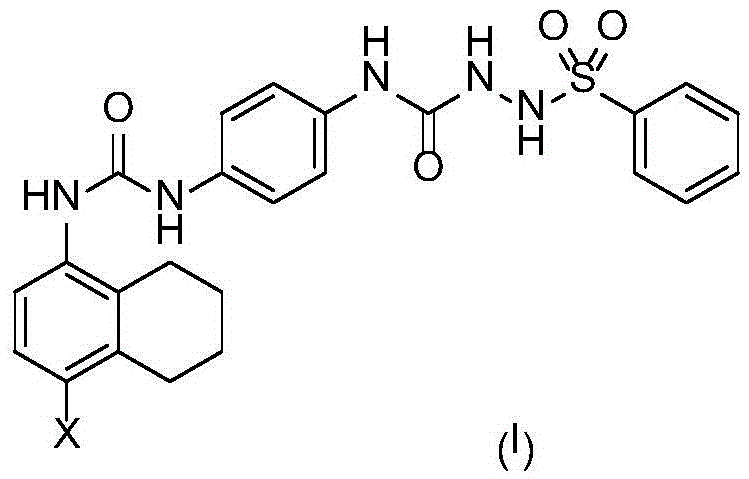
Gpr119 agonist containing benzenesulfonylhydrazide and halogenated benzene structure, preparation method and use thereof
A halogen and aspect technology, applied in metabolic diseases, organic chemistry, drug combination, etc., can solve problems such as unclear exact cause, loss of sensitivity, and decreased insulin secretion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound I-1
[0029]
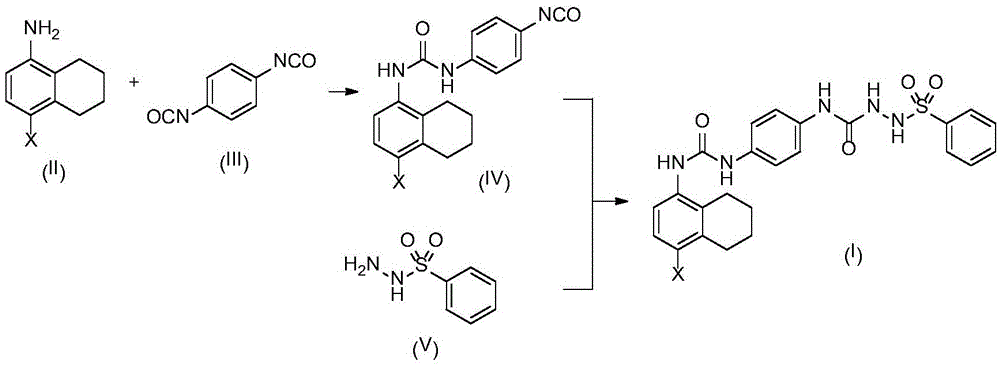
[0030] A. Synthesis of Compound IV-1
[0031] Compound II-11.81g (10mmol) and compound III 4.80g (30mmol) were dissolved in 20mL of dry THF, heated and refluxed for 3 hours, TLC found that the reaction was complete. The reaction mixture was poured into 100 mL ice water with 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-1, a white solid, ESI-MS, m / z=342 ([M+H] + ).
[0032] B. Synthesis of Compound I-1
[0033] Compound IV-10.68g (2mmol) and compound V-10.34g (2mmol) were dissolved in 10mL of dry THF, heated and refluxed for 3 hours, TLC found that the reaction was complete. The reaction mixture was poured into 100 mL ice water wit...
Embodiment 2-4
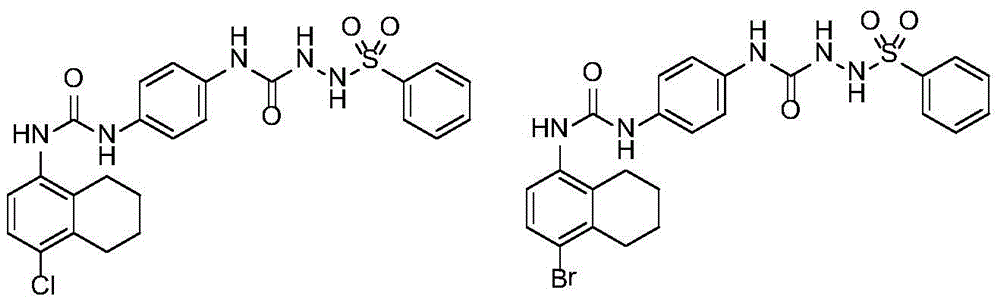
[0035] Referring to the method of Example 1, the compounds listed in the following table were synthesized.
[0036]
[0037]
Embodiment 5
[0038] The preparation of embodiment 5 reference compound D-1
[0039] In order to further illustrate the beneficial effect of the compound of the present invention, the applicant has recorded the compound D-1 and pharmacological data that the applicant has studied but not yet published.
[0040]
[0041] A. Synthesis of Compound IV-5
[0042] Compound II-5 1.47g (10mmol) and Compound III 4.80g (30mmol) were dissolved in 20mL dry THF, heated and refluxed for 3 hours, TLC found that the reaction was complete. The reaction mixture was poured into 100 mL ice water with 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-5, a white solid, ESI-MS, m / z=308 ([M+H] + ).
[0043] B. Synthesis of Compound D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com