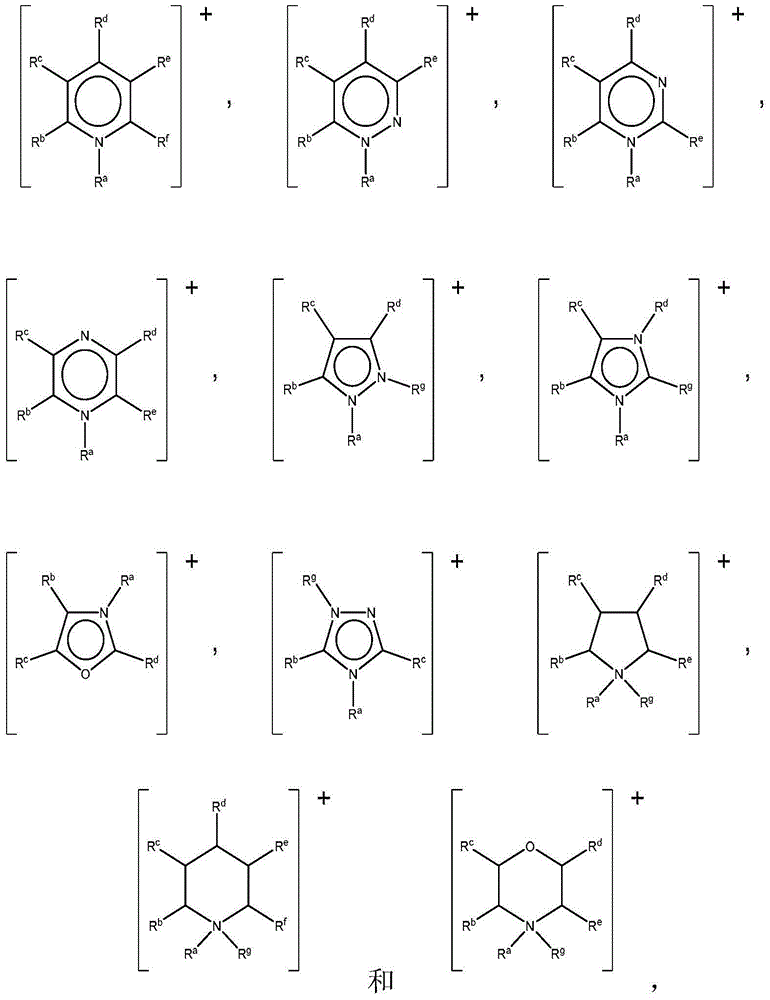
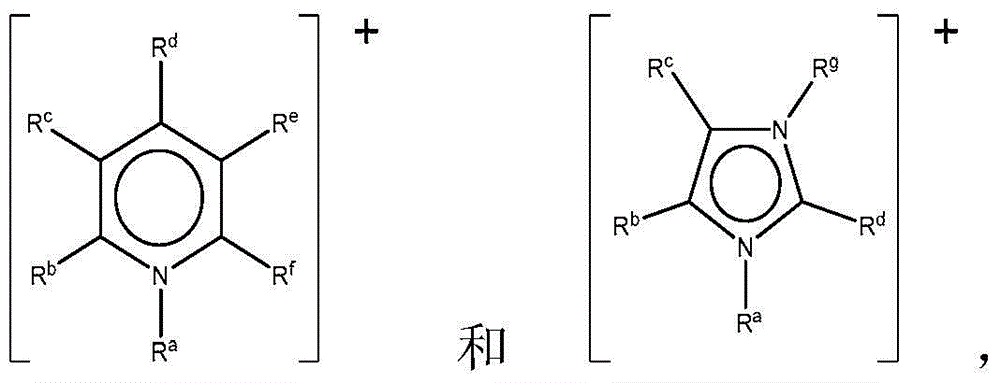
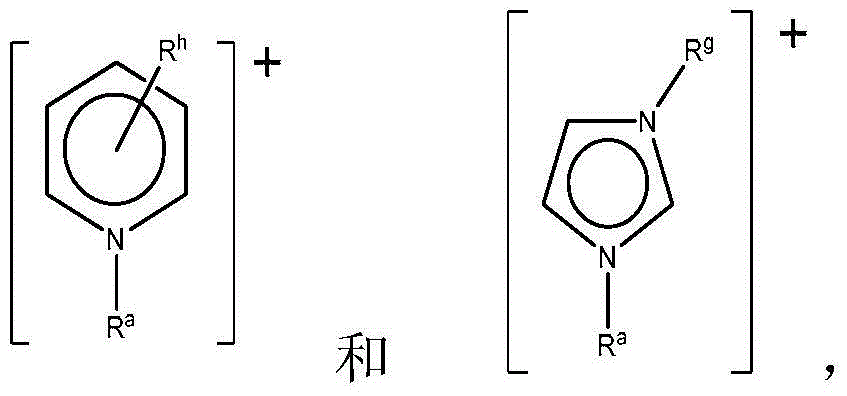
Ionic liquid solvents of perhalide type for metals and metal compounds
An ionic liquid, metal technology, applied in mercury halide, organic chemistry, bulk chemical production, etc., can solve difficult, dangerous and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: the synthesis of perhalide ionic liquid
[0115] Ionic liquids with mixed perhalogen anions ([XY 2 ] - , wherein X=Cl or Br, and Y=Br or I) according to literature method with dialkylimidazolium (dialkylimidazolium), tetraalkylammonium (tetraalkylammonium), tetraalkylphosphonium (tetraalkylphosphonium) and alkylpicoline Onium (alkylmethylpyridinium) cation (see Table 1) preparation. Briefly, liquid bromine or solid iodine is added to an appropriate amount of an organic halide salt to achieve the desired 1:1 molar stoichiometry, yielding a perhalogen anion. The mixture was mixed overnight at room temperature to yield the respective perhalide ionic liquids, which were characterized by mass spectrometry and thermogravimetric analysis (TGA).
[0116] Mass spectrometric identification of cations in each case ([Q] + ) and perhalogen anions ([XY 2 ] - ) in the presence of ionic masses. TGA showed that the volatilization of halogens was significantly reduce...
Embodiment 3
[0122] Example 3: Dissolution of metals
[0123]A series of powdered elemental metal samples (about 0.1 g each, accurately measured) were mixed with 1-butyl-3-methylimidazolium tribromide (1.0 mL) to produce A mixture of approximately 5 wt% metal in the ionic liquid. The mixture was stirred and heated to 60 °C for 72 h, then cooled to room temperature and filtered through a 2 micron nylon filter. For each sample, approximately 0.1 g of filtrate was accurately weighed and digested in 50 ml of 5% w / w nitric acid, and the metal content was determined by inductively coupled plasma (ICP) analysis. The percentage of metal samples dissolved in ionic liquids in each test is shown in Table 2.
[0124] Table 2. Metal Dissolution Test Results
[0125]
Embodiment 4
[0126] Example 4: Qualitative Screening for Mercury Solubilization
[0127] Qualitative screening of the solubility of elemental mercury in ionic liquid systems has been performed by visually observing the state of individual mercury droplets (approximately 0.05 g) stirred in approximately 1-2 mL of ionic liquid at room temperature and 60°C. Complete dissolution under these conditions would result in a mercury loading of about 25-50,000 ppm (about 5 mol%).
[0128] Table 3. Screened perhalide ionic liquids and observations on mercury solubility
[0129]
[0130] a [C 4 mim] + =1-butyl-3-methylimidazolium; [P 6,6,6,14 ] + = Trihexyltetradecylphosphonium; [C 2 C 2 mim] + =1,3-diethylimidazolium; [N 4,4,4,1 ] + = Tributylmethylammonium; [C 4 4 pic] + = 1-butyl-4-methylpyridinium (4-picolinium) (4-picolinium).
[0131] b About 0.05g of mercury is in contact with 1-2mL of ionic liquid, and the mercury solubility is at least 25,000ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com