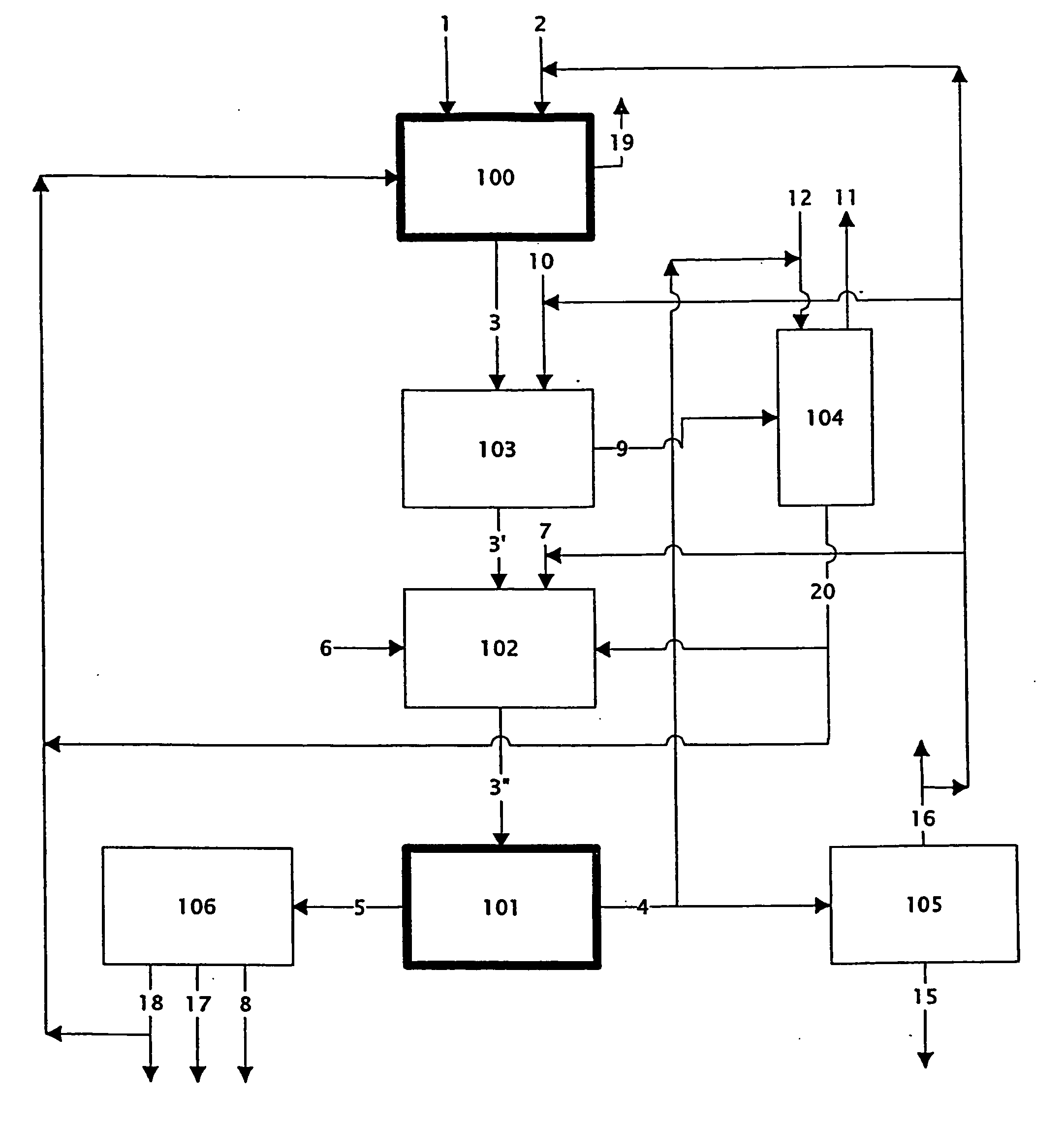
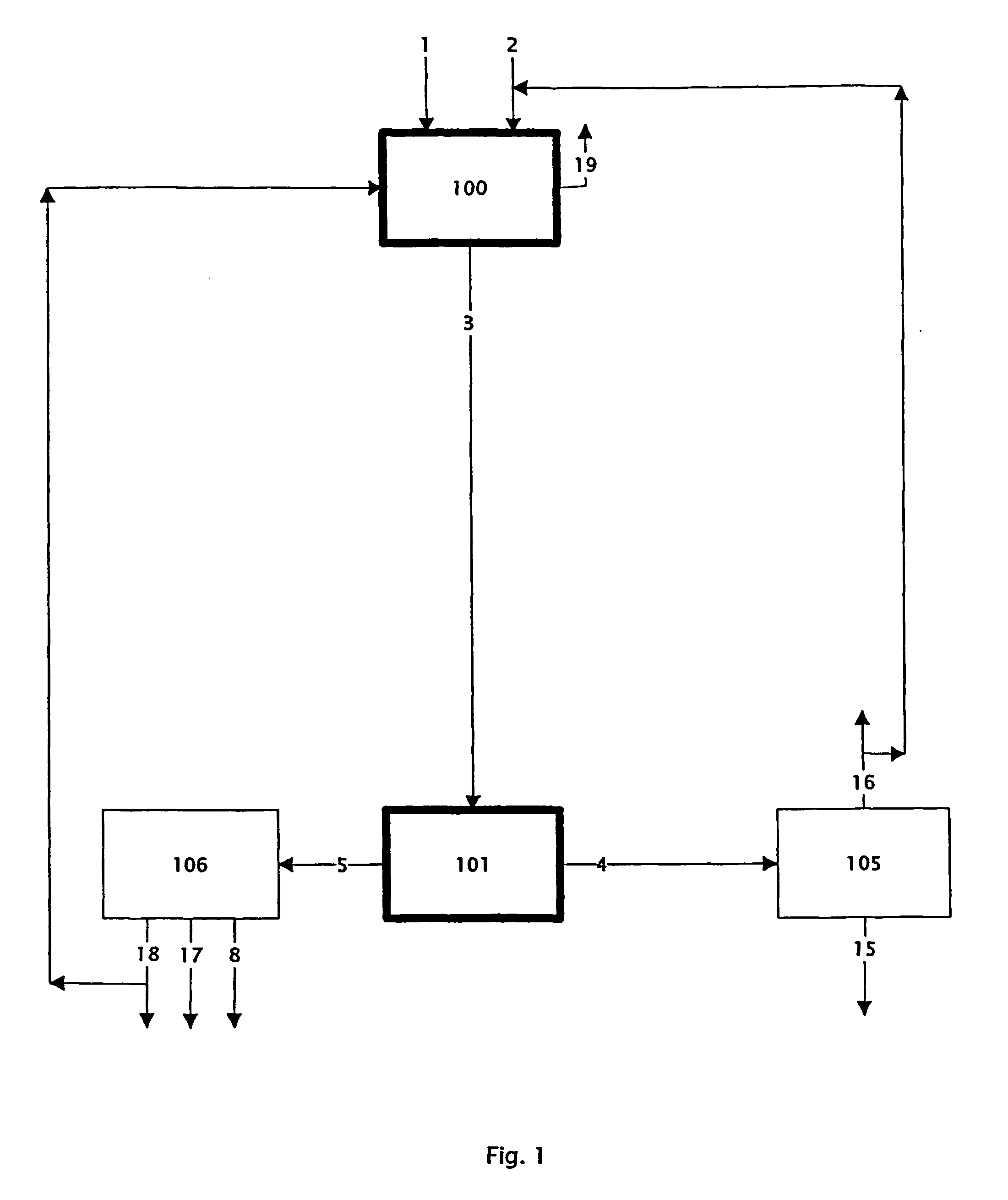
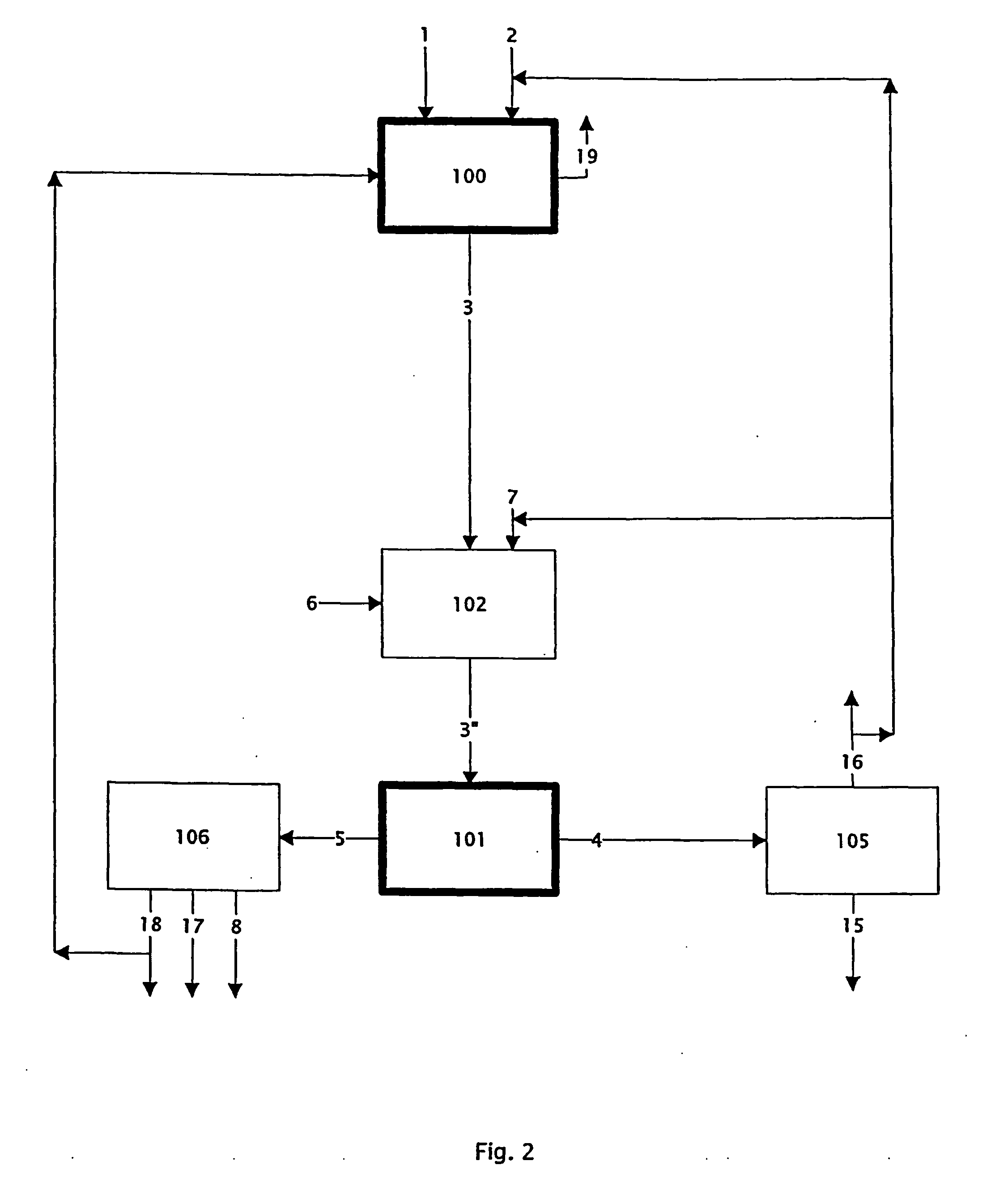
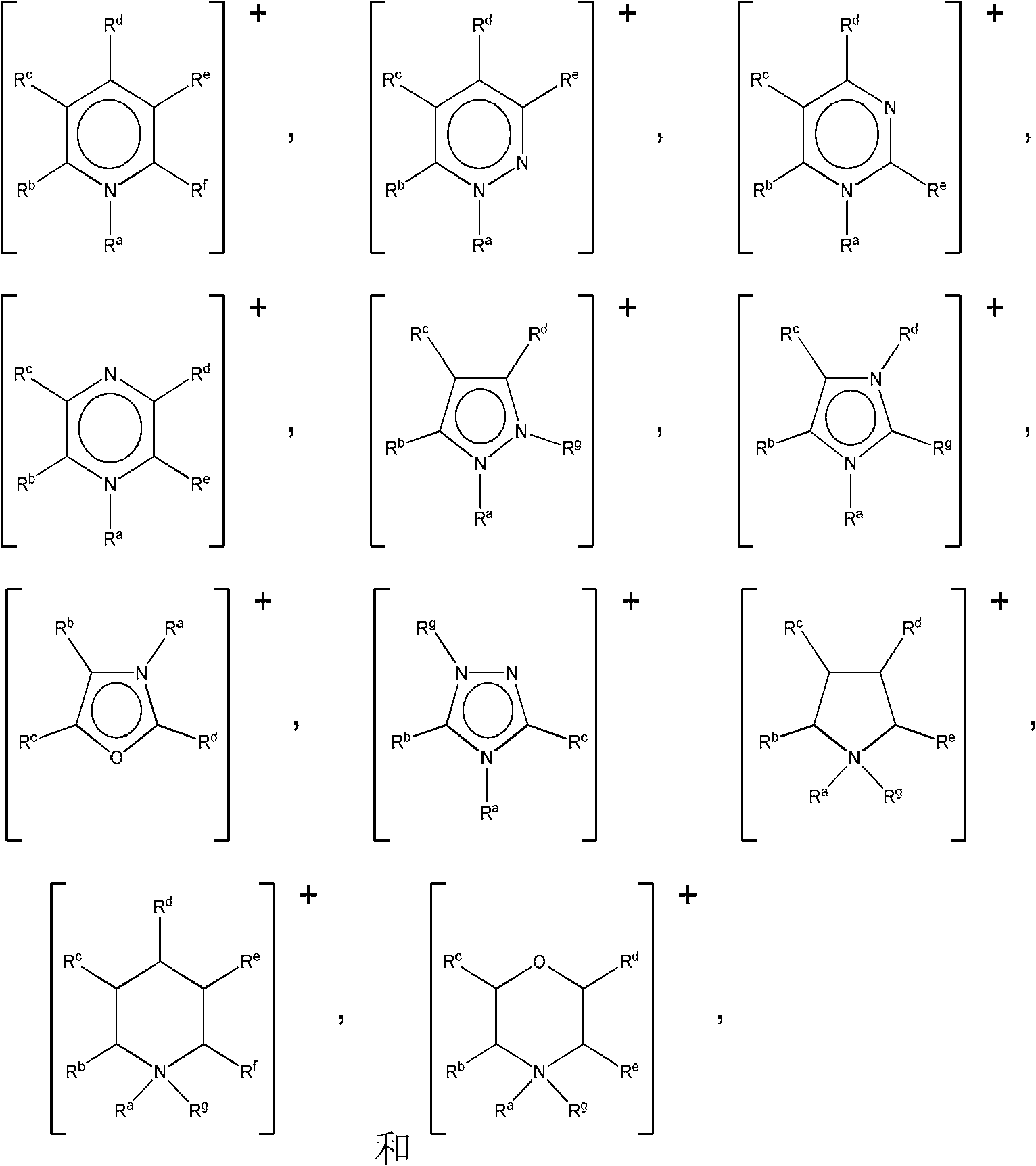
Patents
Literature
57results about "Mercury halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method
ActiveUS20060047132A1High yieldHigh purityZinc halidesGallium/indium/thallium compoundsCompound (substance)Impurity
Owner:UBE IND LTD
Method and apparatus for mitigating mercury emissions in exhaust gases
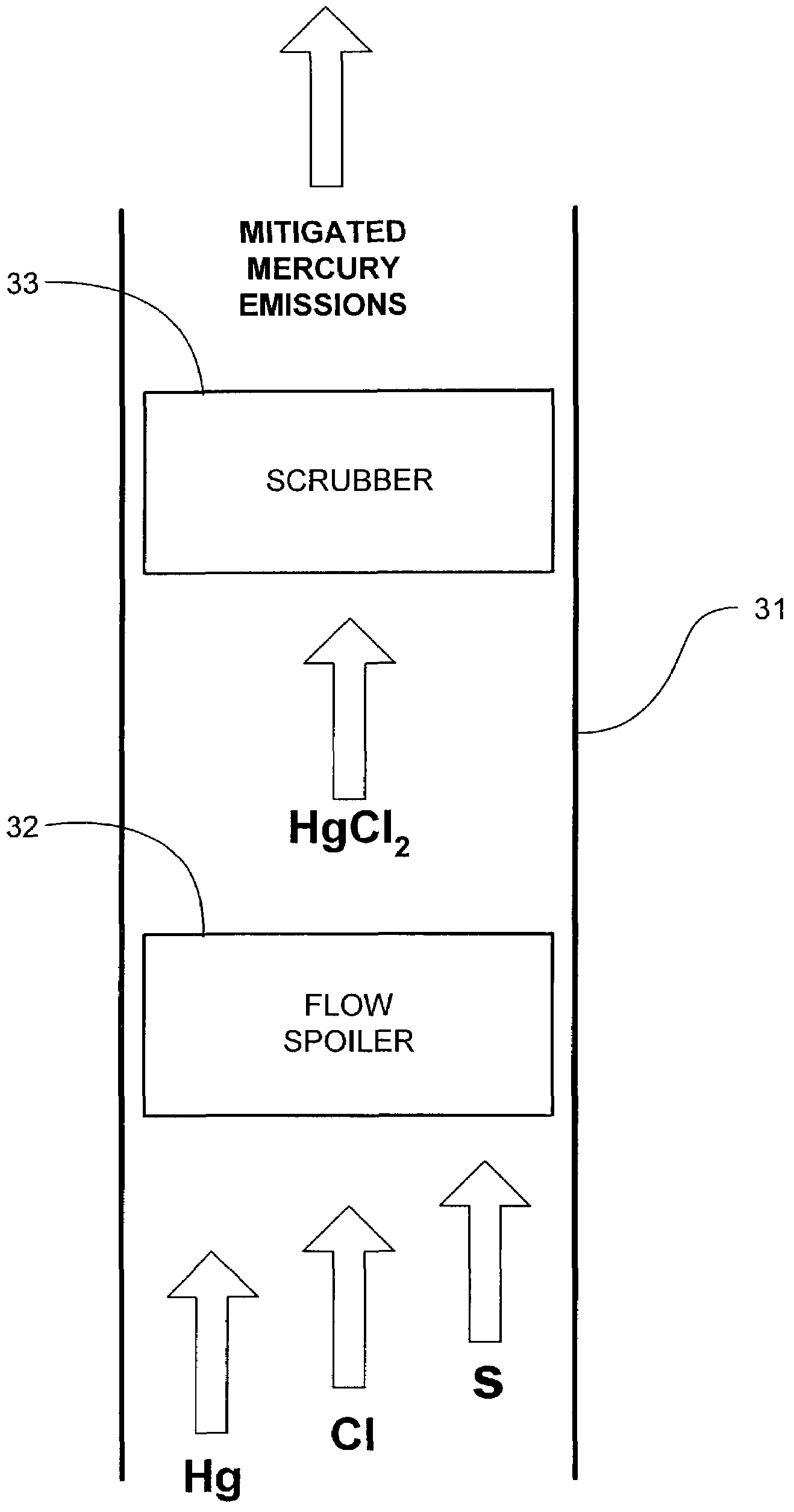
InactiveUS7517511B2Mitigating mercury emissionNitrogen compoundsUsing liquid separation agentMercury DichlorideExhaust fumes
Mercury emissions in an exhaust gas are mitigated. Mercury dichloride is formed upon a surface from a substantial portion of the mercury in the exhaust gas. The mercury dichloride sublimes from the surface, and the sublimed mercury dichloride is subsequently removed from the exhaust stream.
Owner:SCHOFIELD KEITH
Method for making organometallic compounds
Owner:UBE IND LTD
Method for recycling waste mercury catalyst
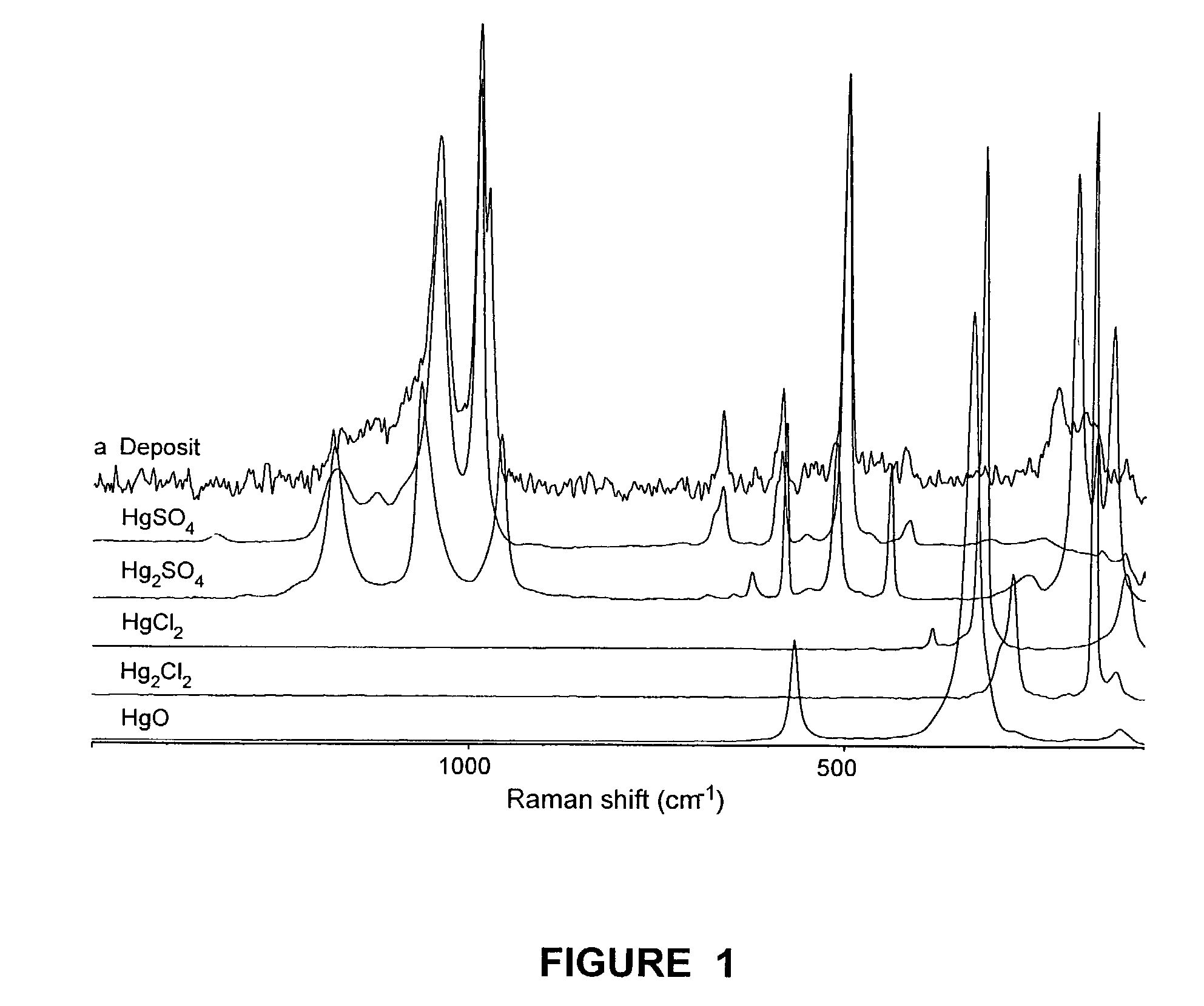
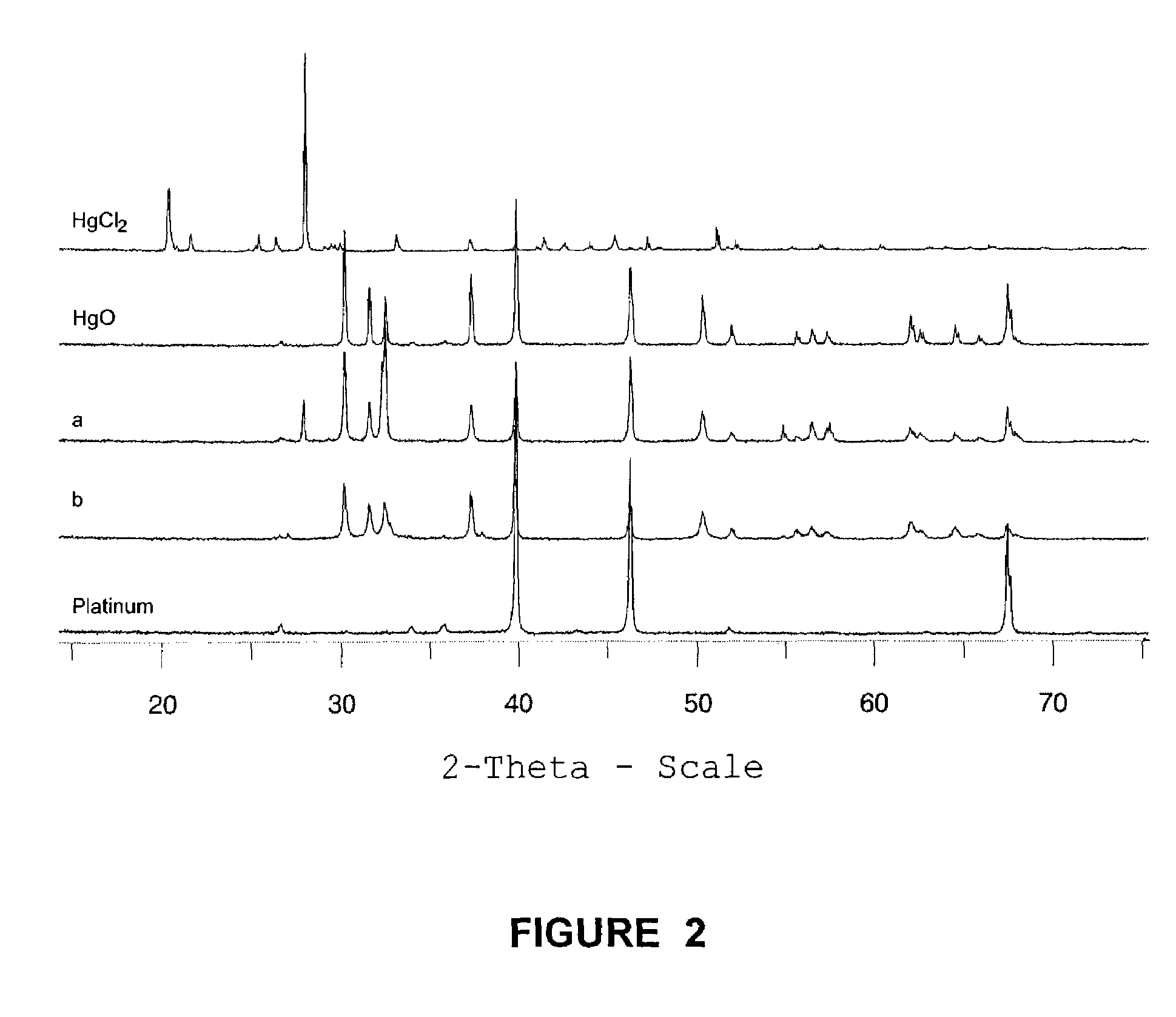
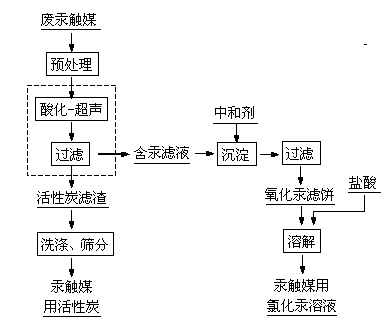
InactiveCN103803638AEfficient use ofEasy to reusePhysical/chemical process catalystsChemical recyclingActivated carbonMercuric ion
The invention discloses a method for recycling a waste mercury catalyst. The method comprises the steps of adding the waste mercury catalyst into water, controlling the temperature at 70-95 DEG C, carrying out hot dissolution pretreatment while stirring, regulating the pH value to 1-3, and filtering after carrying out ultrasonic treatment for 10-60min or filtering in an ultrasonic field to separate mercuric chloride and phosphorus and sulfur impurities from activated carbon in time, wherein the mercuric chloride and the phosphorus and sulfur impurities are separated from the surface of the activated carbon; after filtering, washing the obtained activated carbon and sieving to prepare a mercury catalyst; controlling the temperature of mercury-contained filtrate at 50-90 DEG C, adding a neutralizing agent to regulate the pH value to 6-8, and reacting for 30-90min, wherein mercury ions in the solution are completely converted into mercuric oxide sediments, and toxic substances, namely phosphorus and sulfur in the mercury catalyst still remain in the solution; filtering to obtain a mercuric oxide filter cake, and dissolving with hydrochloric acid to obtain a mercuric chloride solution for preparing the mercury catalyst. According to the invention, the waste mercury catalyst is recycled through acidification-ultrasonic synergistic desorption, coupling and filtration, therefore, the method is simple in process, high in efficiency and low in energy consumption.
Owner:CENT SOUTH UNIV
Method for synchronously recovering mercuric chloride, metal salt and active carbon in spent catalyst
InactiveCN102962033AIncrease profitSolve the scarcityPhysical/chemical process catalystsCalcium/strontium/barium chloridesMetal saltsRaw material
The invention discloses a method for synchronously recovering mercuric chloride, metal salt and active carbon in a spent catalyst. The method is orderly implemented through the steps of spent catalyst drying, mercuric chloride recovery, complex perforation of active carbon and metal salt recovery, wherein mercuric chloride in the spent catalyst is recovered through destructive distillation in combination with condensation and aqueous solution absorption; deposits in the micropores of the active salt are displaced through hot water soaking in combination with bubbling by introducing air, thereby realizing the purpose of recovering the pores of the active carbon; and then the active carbon is recovered through drying and the metal salt is recovered through filtering; in the whole process, no pollutant is generated and discharged and the utilization rate of water is also extremely high; valuable elements in the spent catalyst are all recovered; and the valuable elements in the spent catalyst which is used in production previously and contains mercuric chloride, metal salt and active carbon are utilized, thereby achieving the purposes of solving the problems of scarce raw materials for new catalyst production and environmental pollution caused by inappropriate spent catalyst disposal; as a result, the method is obvious in economic benefit, environmental benefit and social benefit.
Owner:那风换
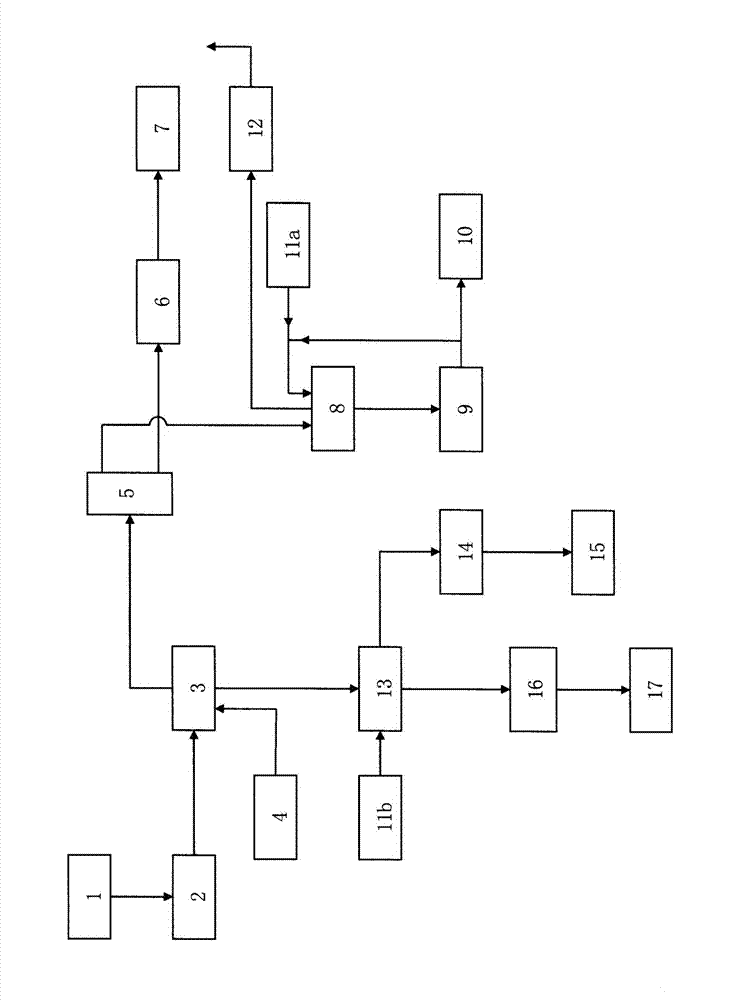
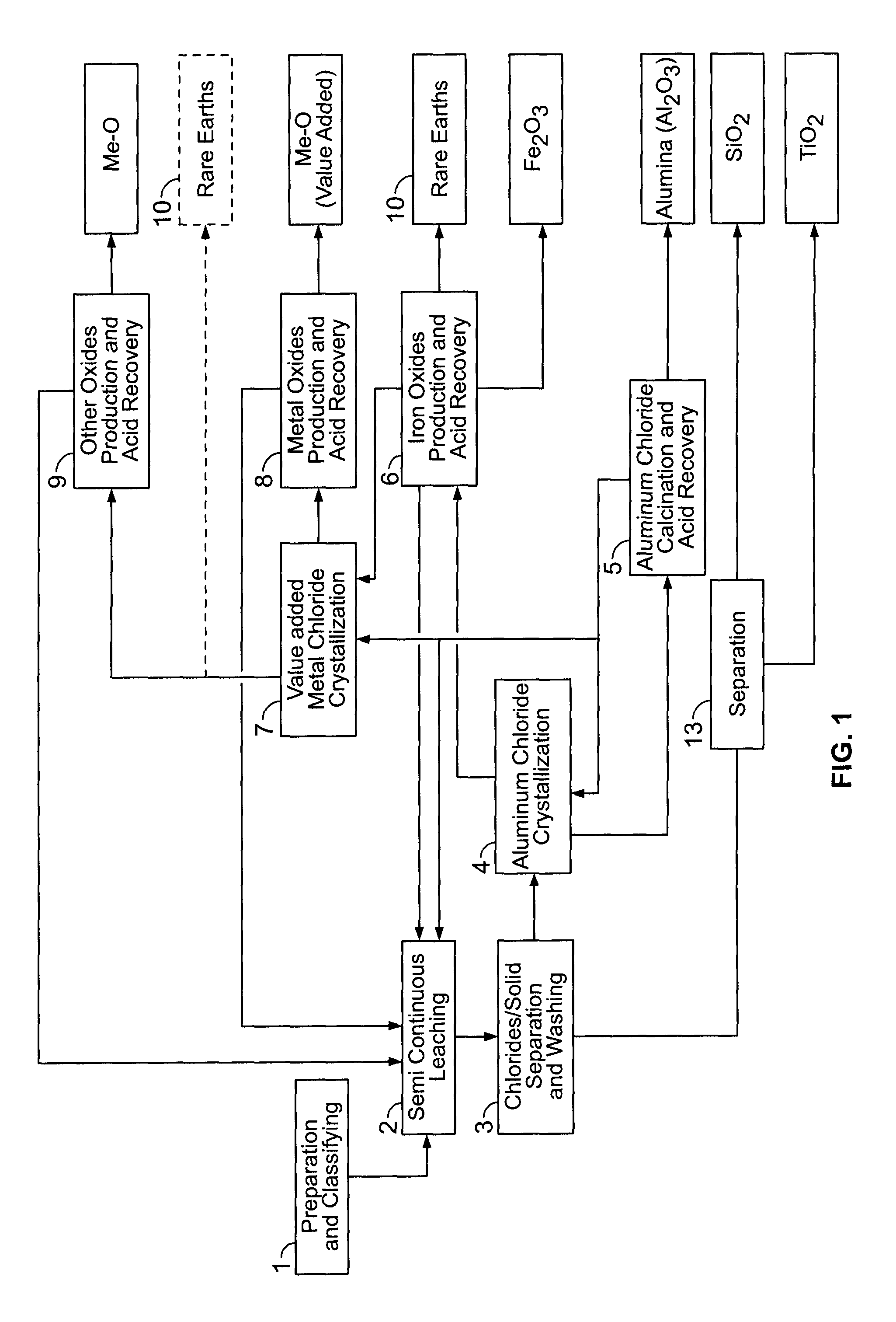
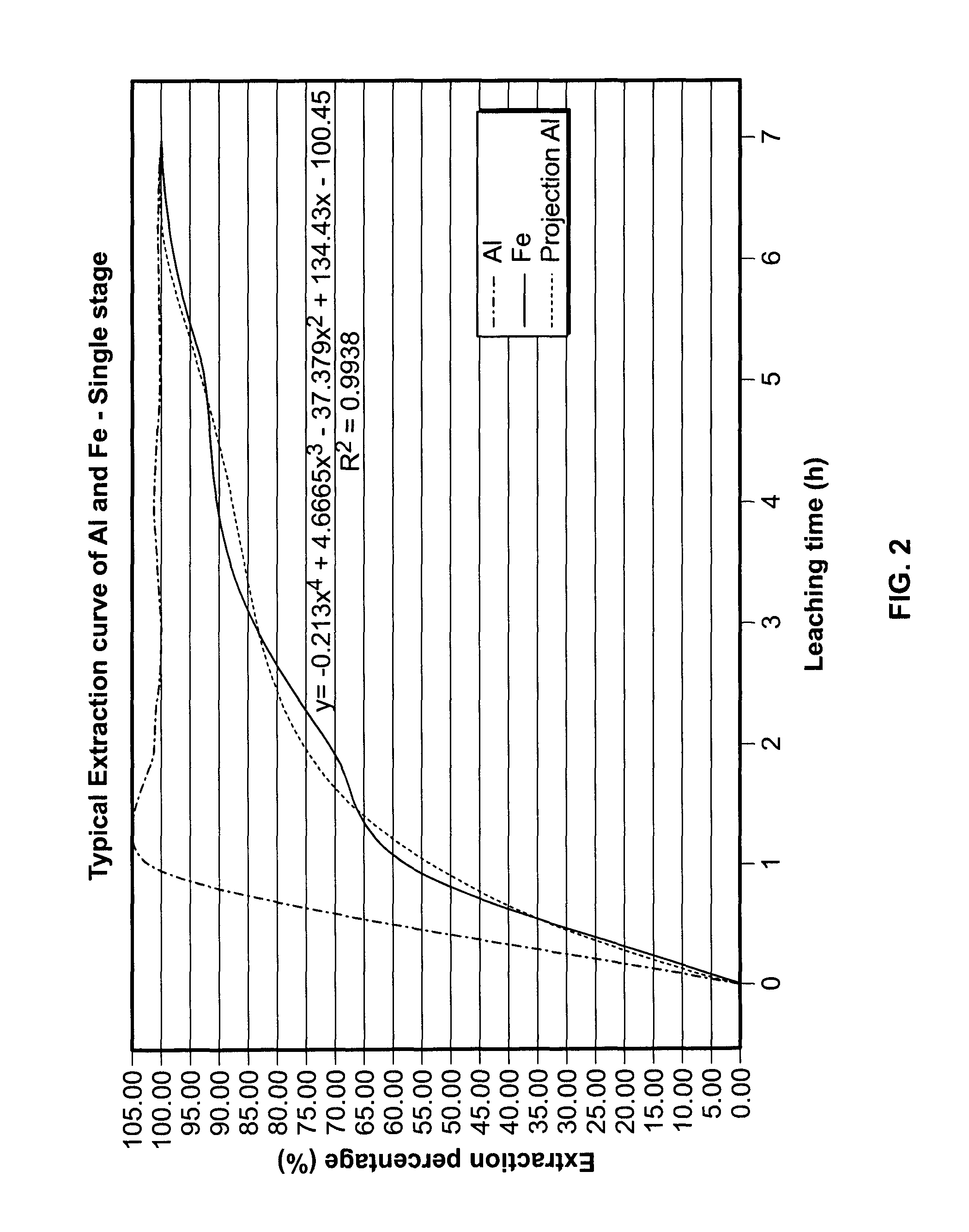
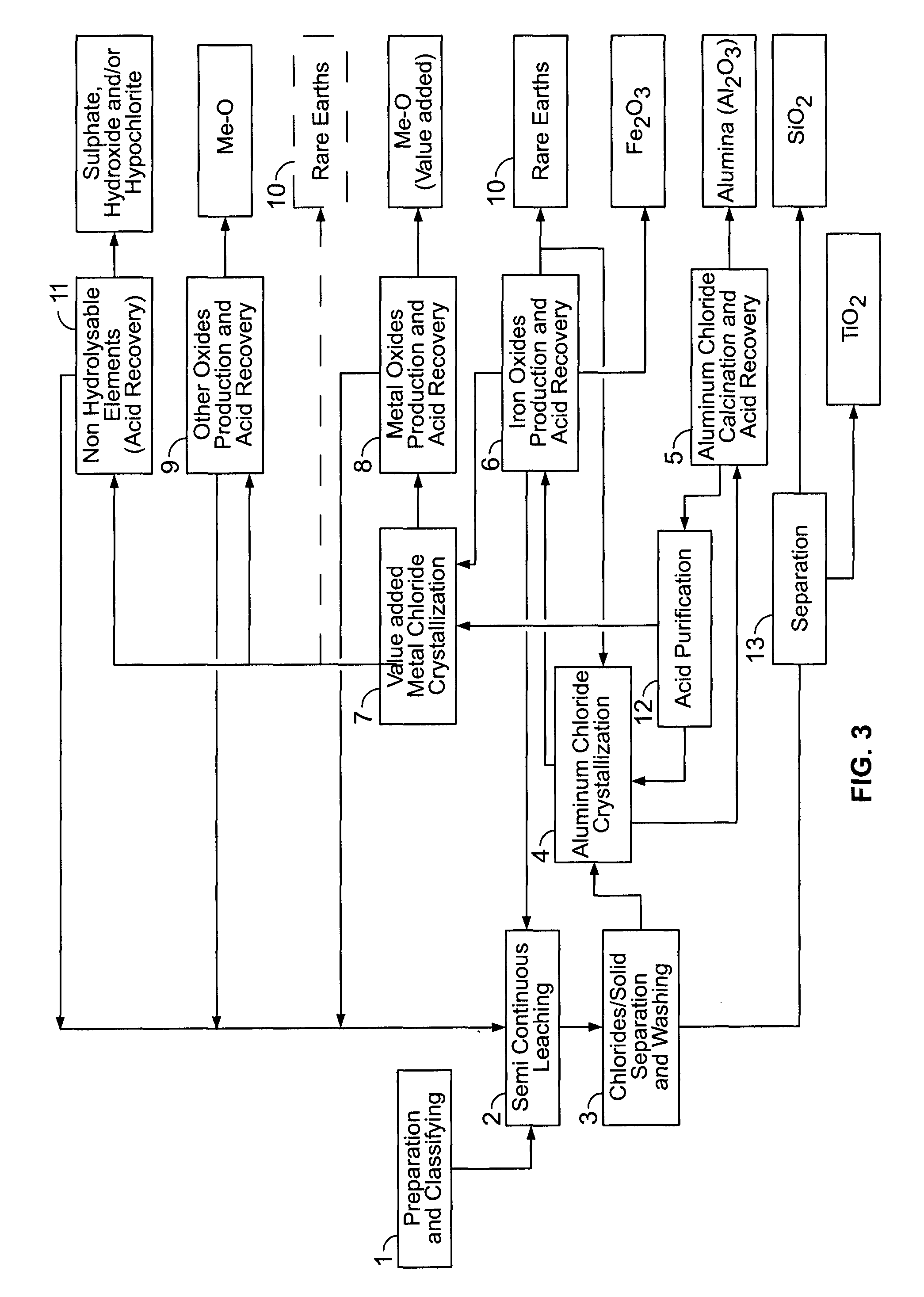
PROCESSES FOR PREPARING ALUMINA AND MAGNESIUM CHLORIDE BY HCl LEACHING OF VARIOUS MATERIALS
The disclosed processes can be effective for treating various materials comprising several different metals. These materials can be leached with HCl for obtaining a leachate and a solid. Then, they can be separated from one another and a first metal can be isolated from the leachate. Then, a second metal can further be isolated from the leachate. The first and second metals can each be substantially selectively isolated from the leachate. This can be done by controlling the temperature of the leachate, adjusting pH, further reacting the leachate with HCl, etc. The metals that can be recovered in the form of metal chlorides can eventually be converted into the corresponding metal oxides, thereby allowing for recovering HCl. The various metals can be chosen from aluminum, iron, zinc, copper, gold, silver, molybdenum, cobalt, magnesium, lithium, manganese, nickel, palladium, platinum, thorium, phosphorus, uranium, titanium, rare earth element and rare metals.
Owner:ORBITE ALUMINAE INC
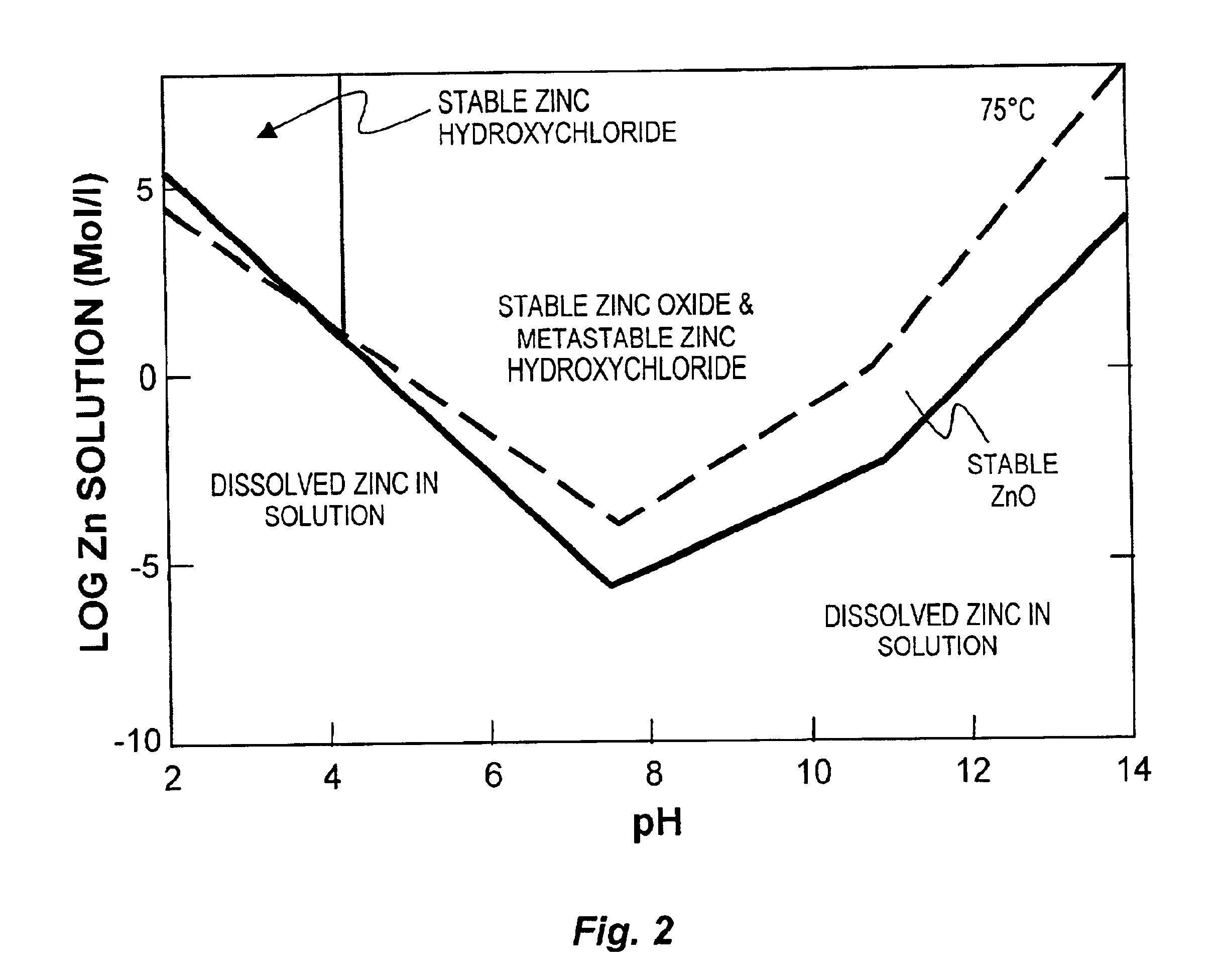
Process to produce simonkolleite, zinc oxide and zinc hydroxide
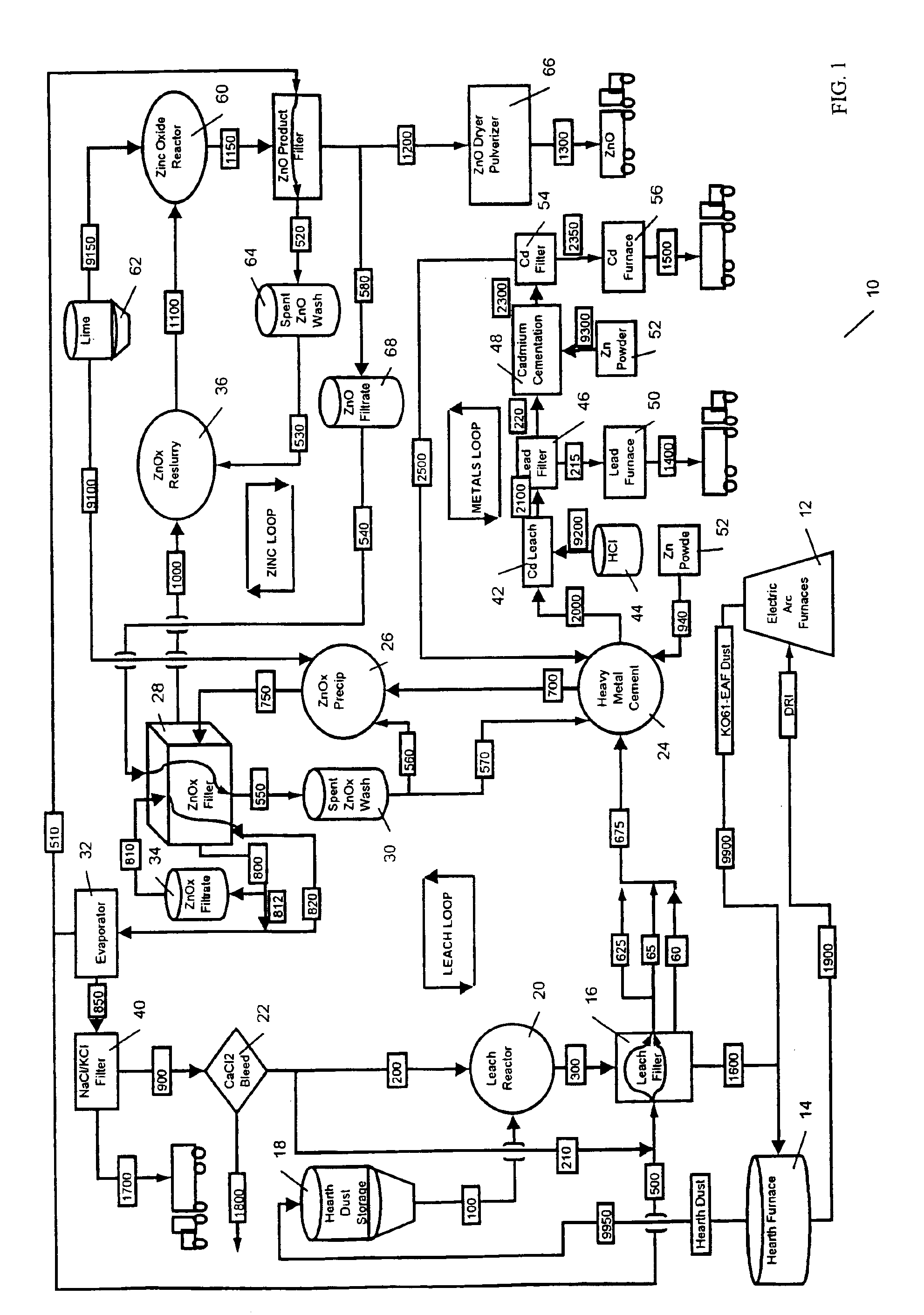
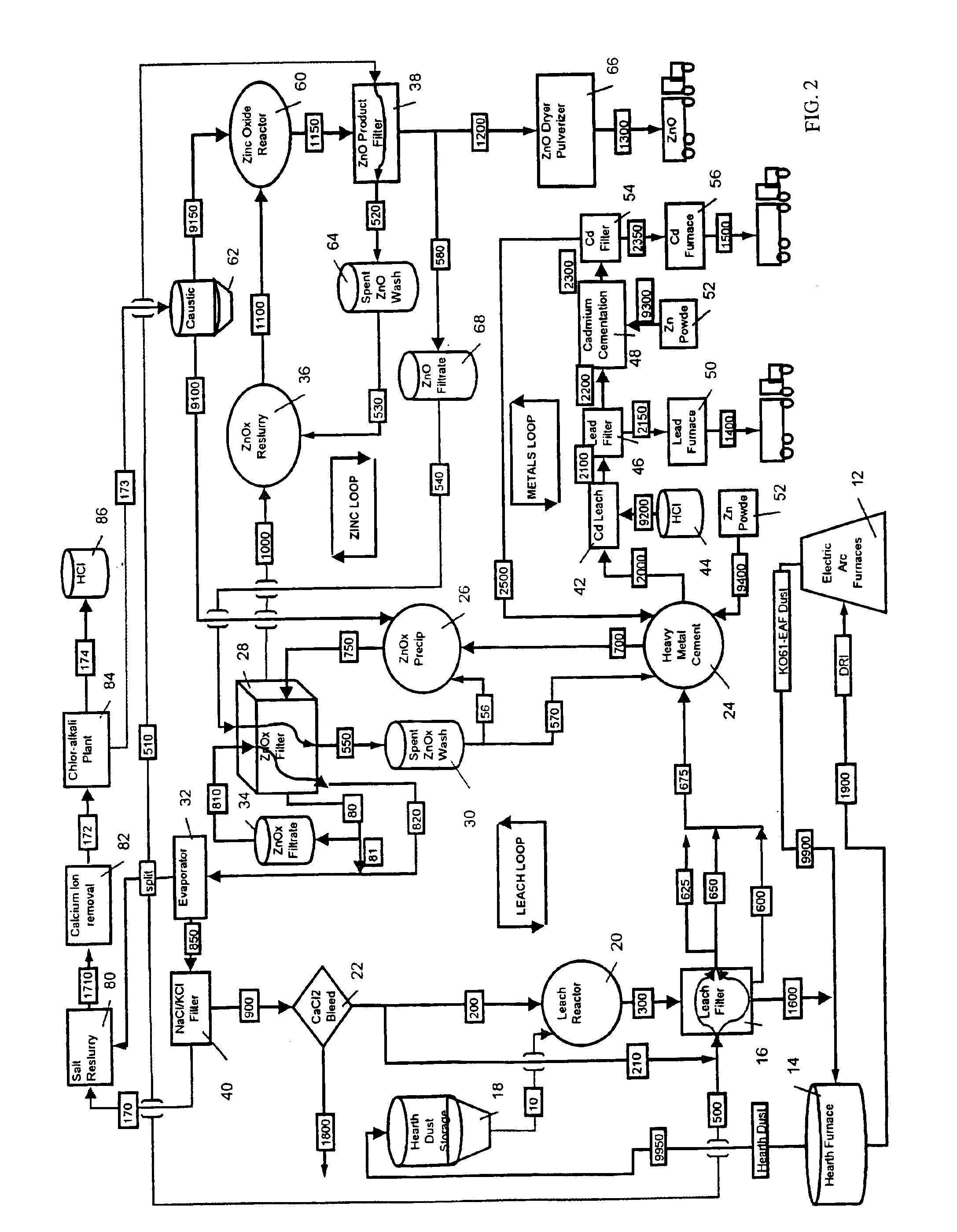
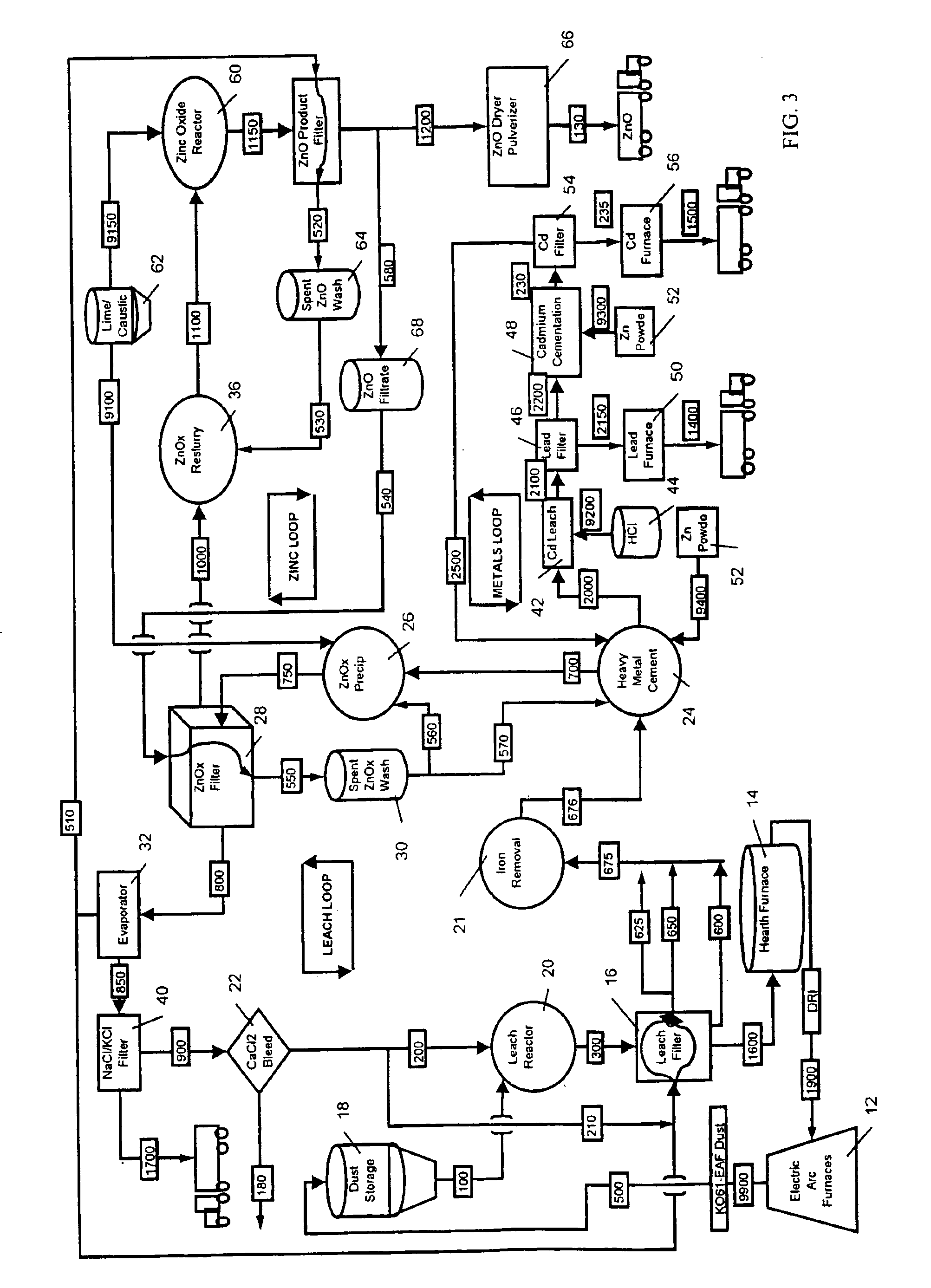
A hydrometallurgical process utilizing an atmospheric calcium chloride leach to selectively recover from various metal feed stocks (consisting of elemental metals, metal oxides, metal ferrite, metal hydroxide, metal carbonates, metal sulfate / sulfur compounds, and their hydrates, specifically including but not limited to EAF Dust K061) zinc, lead, cadmium, silver, copper and other valuable metals to the exclusion of iron, magnesium, halogen salts and other unwanted elements. The process solves the problem of iron and magnesium leach solution contamination because iron is unexpectedly converted to magnetite. The heavy metals are cemented out of solution using zinc or other selected dust at a pH of 6 or greater under unique and unexpected conditions, which do not require acid. Simonkolleite / zinc- oxychloride / zinc-hydroxide is produced from the purified zinc chloride complex pregnant leach solution and is converted directly to high purity active rubber grade 99+% zinc oxide having small particle size and high surface area. The products are metal concentrates suitable for: metal refiner / processors, production of elemental metal, or other conversion processes. The process removes Arsenic and Fluorides in the feed material. The process also solves the problem of chloride contamination in the zinc oxide and prevents heavy metal contaminants in the hydrometallurgically produced zinc oxide derived from feed stocks containing chlorides or when chlorides are used to leach the metal bearing feed stocks. In one embodiment, calcium and / or magnesium compounds are added to the iron bearing waste to increase the recovery of zinc and other non-ferrous metals and to produce an iron bearing flux. The process is environmentally friendly and fully recycles all streams.
Owner:WHITMAN CHESTER W
Method for recycling mercury from mercury-containing solid wastes
InactiveCN102942211AImprove recycling efficiencySimple and fast operationMercury halidesWater chlorinationWastewater
The invention discloses a method for recycling mercury from mercury-containing solid wastes. The method comprises the following steps: introducing N2 (or other inert gases) into mercury-containing wastes at 100 DEG C-200 DEG C and drying for 0.5-10 hours; introducing the chlorine at 200 DEG C-400 DEG C for chlorinating for 0.5-10 hours; introducing N2 again at 400 DEG C-750 DEG C and dry distilling for 1-10 hours; and lastly, washing with a solvent, thereby obtaining a product, and meanwhile, absorbing the dry distilling tail gas by using absorption liquid. According to the method, the recovery rate of the mercury can reach 80-99%; the recycled waste mercury bichloride contact agent carrier is not damaged; the mercury content is extremely reduced to below 0.1% and the recycled waste mercury bichloride contact agent carrier still can be applied to the production of a mercury bichloride contact agent; the pollution problem of the mercury-containing waste and the recycling problem of the mercury resource are solved according to the method; the traditional mercury recycling method and the mercury bichloride production are integrated; the production cost is saved; and the method has the advantages of zero remnant of waste residue and waste water, simpleness in technology, low cost, waste recycling and environmental protection.
Owner:XINJIANG TIANYE GRP +1
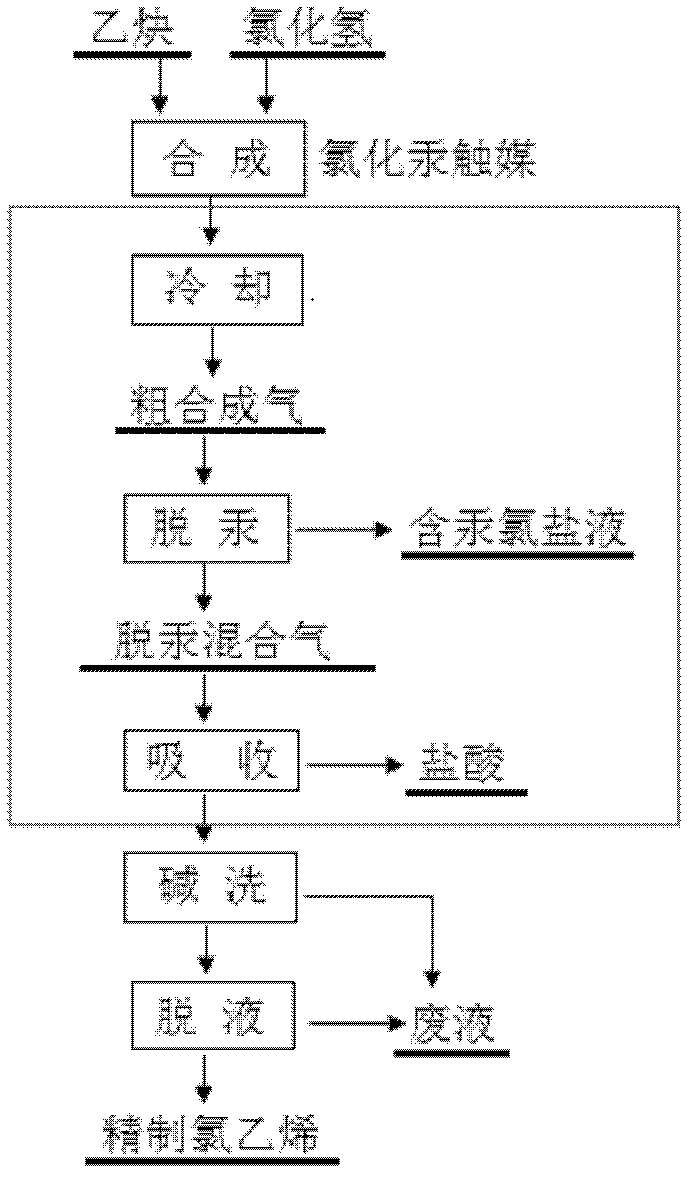
Comprehensive processing method of vinyl chloride synthetic gas
ActiveCN102516022AHigh removal rateShort processChlorine/hydrogen-chloridePreparation by halogen halide additionHigh concentrationSyngas
The invention discloses a comprehensive processing method of vinyl chloride synthetic gas. Under the effect of a mercuric chloride catalyst, acetylene gas and hydrogen chloride are subject to a reaction, such that crude vinyl chloride synthetic gas is produced; the crude vinyl chloride synthetic gas is subject to mercury removing by using a high-concentration chloride containing hydrochloric acid; hydrogen chloride is absorbed by using water; the crude vinyl chloride synthetic gas is then subject to steps such as alkali washing and dehydration, such that refined vinyl chloride is obtained, and requirements of mercury recovering, qualified industrial hydrochloric acid production and vinyl chloride refining are directly achieved.
Owner:CENT SOUTH UNIV
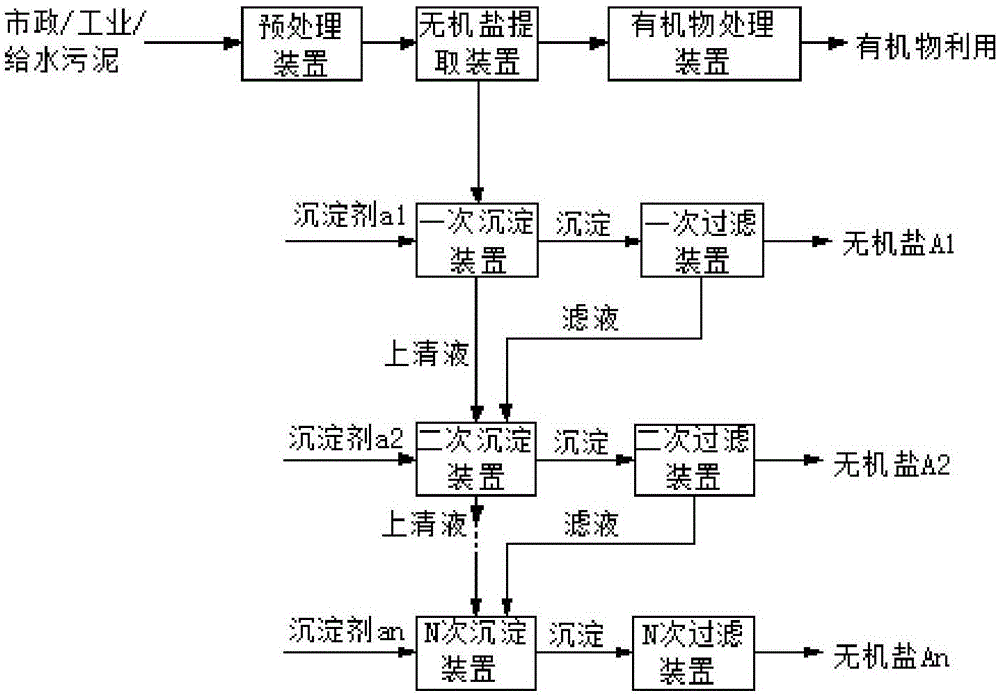
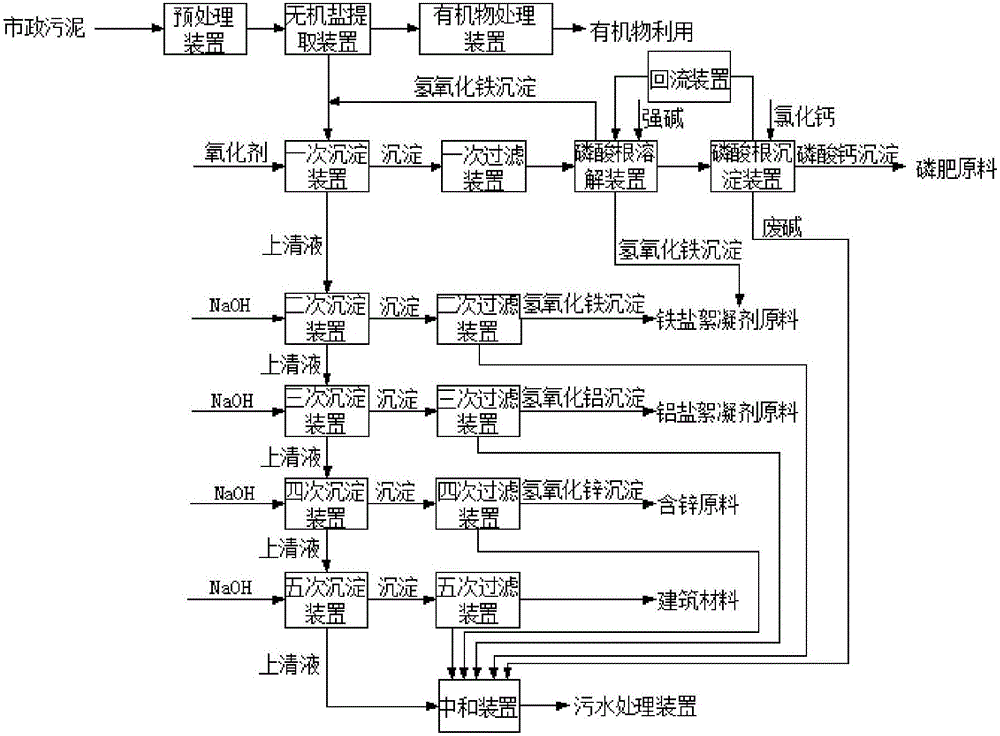
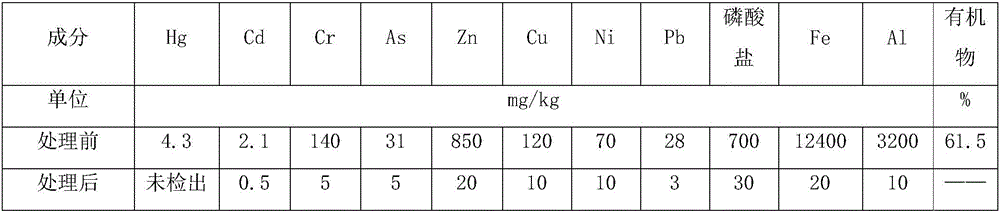
Method and device for respectively extracting and using inorganic matters in sludge
InactiveCN106007292ALow impurity contentHigh puritySludge treatmentLead hydroxidesInorganic saltsResource utilization
The invention provides a method for respectively extracting and using inorganic matters in sludge, and belongs to the technical field of sludge treatment. The method comprises the following steps: (1) pretreating the sludge; (2) extracting inorganic salt from a sludge solution subjected to pretreatment; (3) adding a precipitant a1 into the inorganic salt extracting solution obtained in the step (2) till precipitates are separated, and forming inorganic salt A1 for recycling; (4) adding a precipitant a2 into precipitate-removed supernate in the step (3) till precipitates are separated, and forming inorganic salt A2 for recycling; (5) by parity of reasoning, adding a precipitant an into the precipitate-removed supernate in the former step till precipitates are separated, forming inorganic salt An for recycling. According to the method, the inorganic matters in the sludge are respectively recycled, so that weight reduction for the sludge is realized, and resource utilization of the sludge is also realized; the method is simple in process, high in applicability, simple and convenient to operate, easy to control, low in operating cost, good in treatment effect, wide in application range and easy to popularize and use.
Owner:TIANJIN ENEW ENVIRONMENTAL PROTECTION ENGCO LTD
Chloride melt process for the separation and recovery of zinc
InactiveUS20050006247A1Easy to controlIncrease flexibilityZinc halidesChlorine/hydrogen-chlorideBoiling pointChloride
Process for the production of ZnCl2 from a Zn bearing primary and / or secondary material comprising the steps of reacting the Zn bearing material with a chlorinating agent such as Cl2 to convert metals into chlorides and vaporising the volatile components of the reaction product at a temperature between the melting point of said reaction product and the boiling point of ZnCl2, thereby recovering a Zn rich chlorinated melt, and thereafter distilling ZnCl2 from this Zn rich chlorinated melt, thereby recovering purified ZnCl2 and a Zn-depleted chlorinated melt.
Owner:UMICORE AG & CO KG
Ionic liquid solvents of perhalide type for metals and metal compounds
The present invention relates to a process for dissolving metals in perhalide containing ionic liquids, and to the extraction of metals from mineral ores; the remediation of materials contaminated with heavy, toxic or radioactive metals; and to the removal of heavy and toxic metals from hydrocarbon streams.
Owner:REPSOL SA
Reduction of zinc oxide from complex sulfide concentrates using chloride processing
InactiveUS6843976B2Reduce Chloride ContentZinc halidesSolvent extractionPregnant leach solutionSulfide
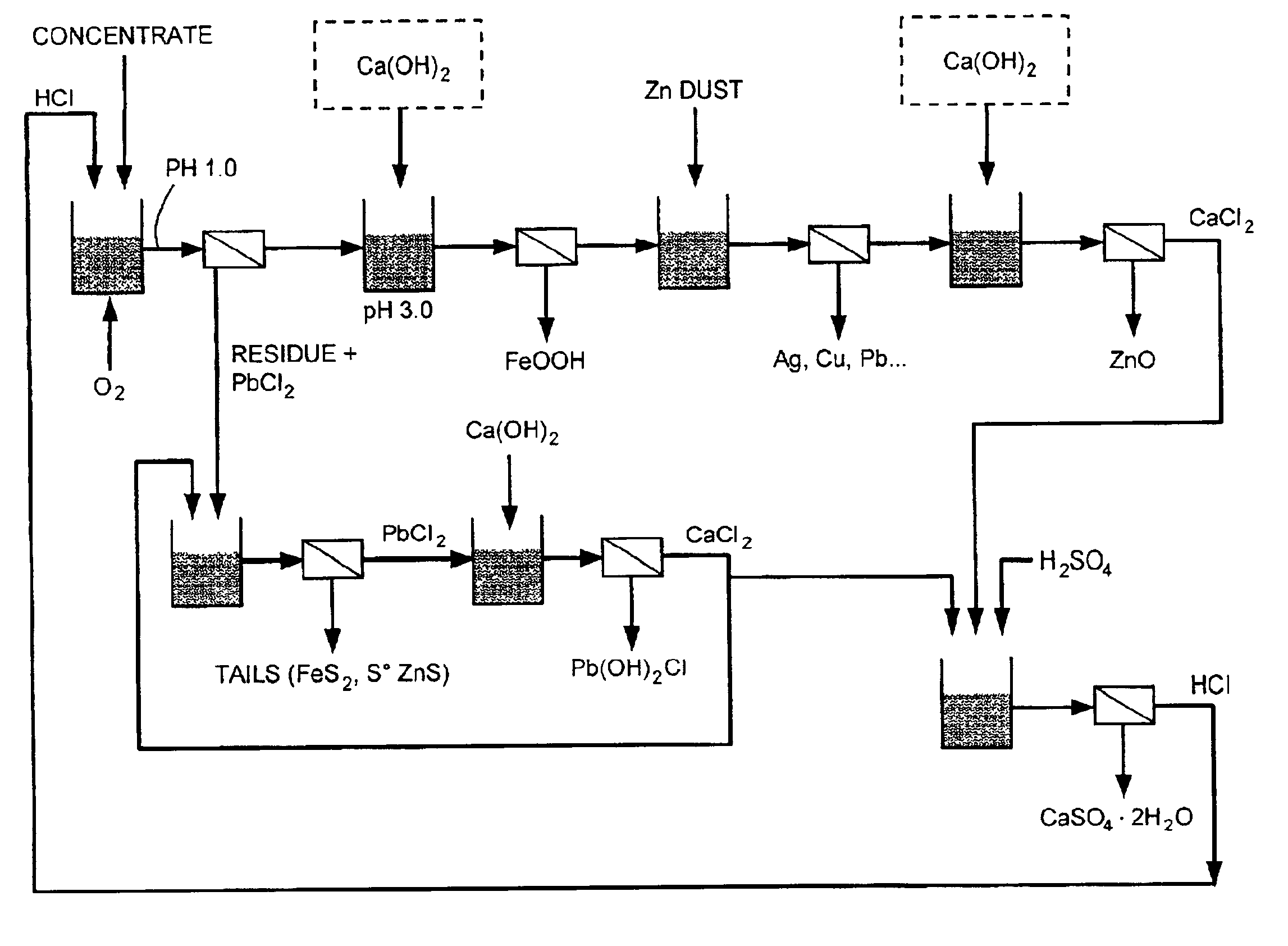
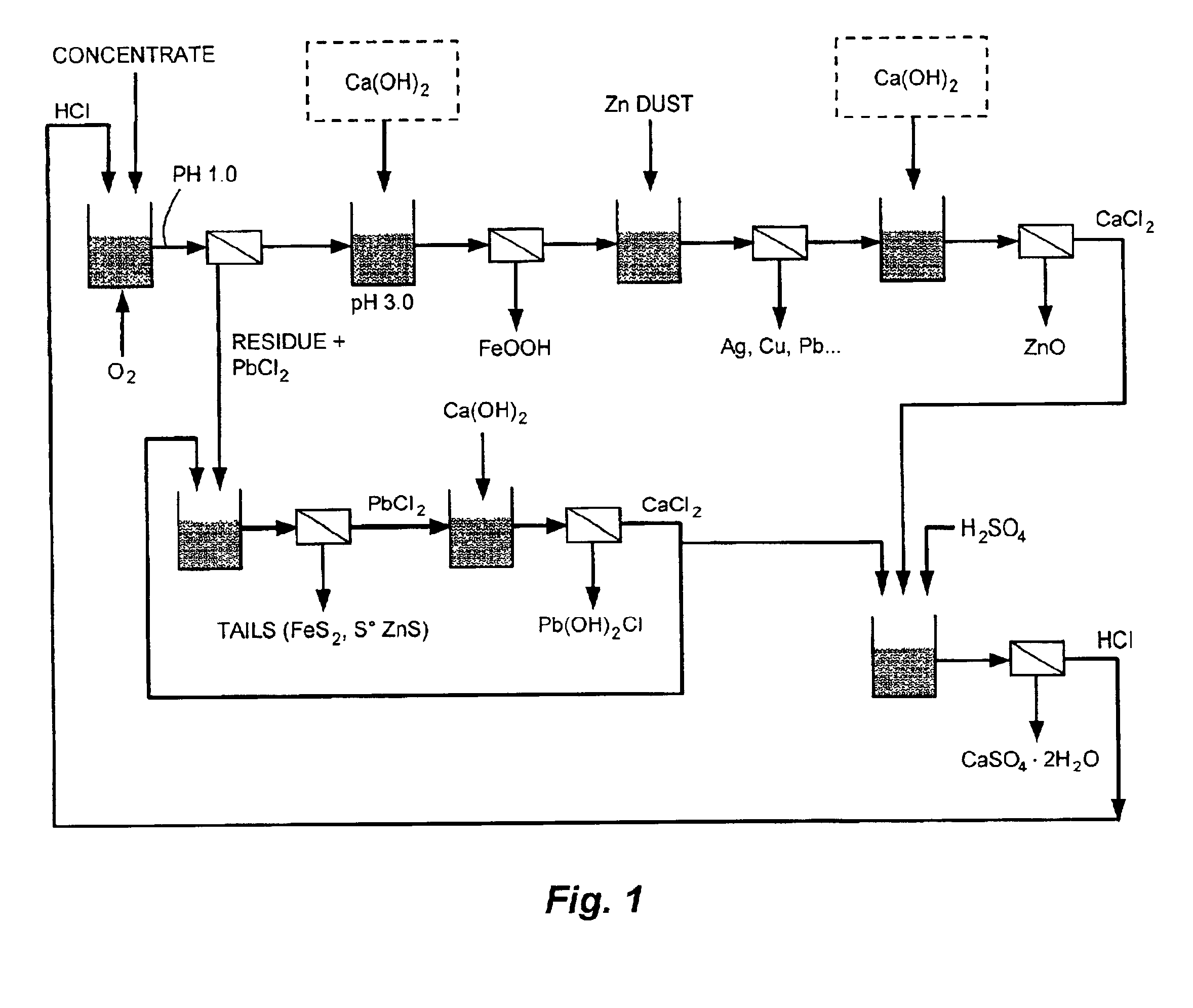
An apparatus and a process for producing zinc oxide from a zinc-bearing material are provided. The process comprises the steps of leaching the complex sulfide material with hydrochloric acid, ferric chloride, and oxygen; precipitating iron from the leach solution using lime and oxygen; removing copper, silver, cadmium, cobalt and lead from the leach solution by cementation with zinc dust; precipitating zinc oxide from the leach solution using lime; and regenerating HCl from a calcium chloride leach filtrate solution to regenerate hydrochloric acid and precipitate gypsum. Related processes for recovering copper, silver, lead, and iron from complex sulfide materials and for recovering lead from residue by solubilizing lead chloride and precipitating it with lime are also provided.
Owner:FALCONBRIDGE LTD
Processing method of chlorine-containing tail gas produced by mercuric chloride production
ActiveCN104084026APromote decompositionGood catalyticDispersed particle separationMercury halidesChlorine dioxideReduction treatment
The invention discloses a processing method of chlorine-containing tail gas produced by mercuric chloride production. The processing method comprises the following steps: producing a sodium hypochlorite solution from tail gas which contains chlorine and mercuric chloride vapor, and then adding nickel chloride (or nickel oxide or nickel hydroxide) for nickel oxide hydroxide generating reaction; mixing sodium hypochlorite with nickel oxide hydroxide in a decomposition reaction kettle for two to three hours; performing coexistence of a solution from the decomposition reaction kettle with nickel oxide hydroxide in a continuous decomposition tank for three to five days, wherein when the pH value approaches to seven to eight, decomposition is completed; then putting a sodium chloride solution into a soluble alkali trough, adding alkali until the concentration of sodium hydroxide reaches 5% to 10%, and then returning to an absorption reaction kettle to be used again; sucking chlorine dioxide, chlorine and hydrochloric acid gas which are decomposed everywhere into a reduction treatment tower by negative pressure generated by a fan to enable the gases to contact with alkali liquor, wherein sodium chloride returns to an alkali liquor precipitation tank, and gases are exhausted by an induced draft fan. With the processing method, stable by-products can be obtained, highly toxic mercury chloride can be recycled, and the up-to-standard release of tail gas can be realized.
Owner:贵州重力科技环保股份有限公司
Ionic liquid solvents of perhalide type for metals and metal compounds
The present invention relates to a process for dissolving metals in perhalide containing ionic liquids, and to the extraction of metals from mineral ores; the remediation of materials contaminated with heavy, toxic or radioactive metals; and to the removal of heavy and toxic metals from hydrocarbon streams.
Owner:REPSOL SA
Method using microwave heating to treat mercury-containing acid sludge
PendingCN109136566AEfficient recyclingOvercome efficiencyProcess efficiency improvementMercury halidesActivated sludgeMicrowave
The invention discloses a method using microwave heating to treat mercury-containing acid sludge and belongs to the technical field of solid waste treatment and recycling. The method includes: sequentially subjecting the mercury-containing acid sludge to drying and microwave heating under inert gas protection to obtain HgS-containing acid sludge and HgCl2 steam; subjecting the HgCl2 steam to condensation and dissolving with water to obtain a mercuric chloride solution for a mercury waste medium; subjecting the HgS-containing acid sludge to microwave calcining in oxygen atmosphere to obtain elemental mercury steam, and subjecting the obtained Hg steam to condensation to obtain pure elemental mercury. The method has the advantages by subjecting the mercury-containing acid sludge to microwaveheating, mercuric chloride and the elemental mercury can be efficiently recycled, and the method is simple in process flow, clean and environmentally friendly, cheap in raw materials and huge in environmental benefits and economic benefits.
Owner:KUNMING UNIV OF SCI & TECH
Chloride melt process for the separation and recovery of zinc
InactiveUS6921474B2Easy to controlIncrease flexibilityZinc halidesPhotography auxillary processesBoiling pointChloride
Process for the production of ZnCl2 from a Zn bearing primary and / or secondary material comprising the steps of reacting the Zn bearing material with a chlorinating agent such as Cl2 to convert metals into chlorides and vaporising the volatile components of the reaction product at a temperature between the melting point of said reaction product and the boiling point of ZnCl2, thereby recovering a Zn rich chlorinated melt, and thereafter distilling ZnCl2 from this Zn rich chlorinated melt, thereby recovering purified ZnCl2 and a Zn-depleted chlorinated melt.
Owner:UMICORE AG & CO KG
Method for synthesizing mercurous iodide
InactiveCN103553118AReliable raw materialsThe stoichiometric ratio does not have a substantial effectMercury halidesReduction rateIodide
The invention discloses a method for synthesizing mercurous iodide. The method comprises the steps of: S1, cleaning and drying an ampoule for future use; S2, putting the measured mercury and mercury iodide into the ampoule prepared in the step S1, vacuumizing the ampoule and sealing the ampoule when the vacuum degree is reduced to the range from 10<-2> to 10<-3> Pa; S3, vertically fixing the ampoule in the step S2 in a reaction furnace, and increasing the temperature of the reaction furnace to the range from 300 to 370 DEG C at a temperature rise rate of 20-100 DEG C per hour; S4, after all the mercury iodide is melted, standing for 1-4 h; S5, reducing the temperature of the reaction furnace to any temperature under the syntectic reaction temperature of an iodine-mercury binary system and above the eutectic reaction temperature of the iodine-mercury binary system at a temperature reduction rate of 200-400 DEG C per hour, preserving heat for 2-10 h and then cooling to the room temperature; and S6, taking out the obtained product in the ampoule and removing mercury at the bottom and mercury iodide at the top. The method for synthesizing the mercurous iodide is capable of obtaining the mercurous iodide in which the ratio of mercury atoms to iodine atoms is 1: 1; and the crystallization temperature range is wide and the method is easy to control.
Owner:XIHUA UNIV
Application of mercuric iodobromide as infrared band second-order nonlinear optical material
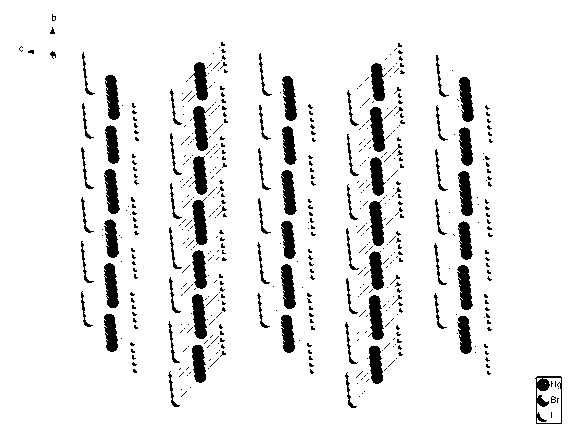
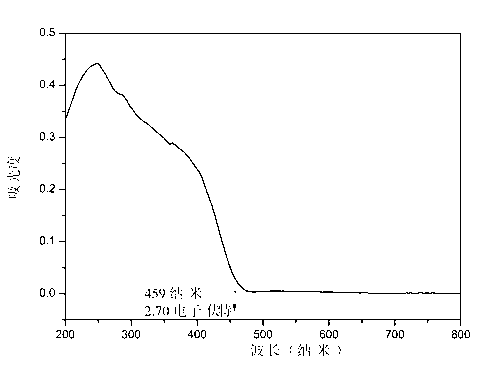
InactiveCN103073050AWide band of light transmissionLarge second-order nonlinear optical coefficientPolycrystalline material growthFrom normal temperature solutionsNonlinear optical crystalSpace group
The invention discloses a novel second-order nonlinear optical material. The molecular formula of the novel second-order nonlinear optical material is HgBrI; and a crystallographic space group is Cmc21; a is 4.673(2); b is 7.130(3); c is 13.314(6); alpha is 900, beta is 900, gamma is 900 and Z is 4. The novel second-order nonlinear optical material has remarkable characteristics of strong second-order nonlinear optical effect which can be matched by phase, large light-transmitting windows in a visible light zone and an infrared light zone and favorable thermal stability. The crystal material can be widely applied to the fields of optics and the like.
Owner:WUHAN UNIV
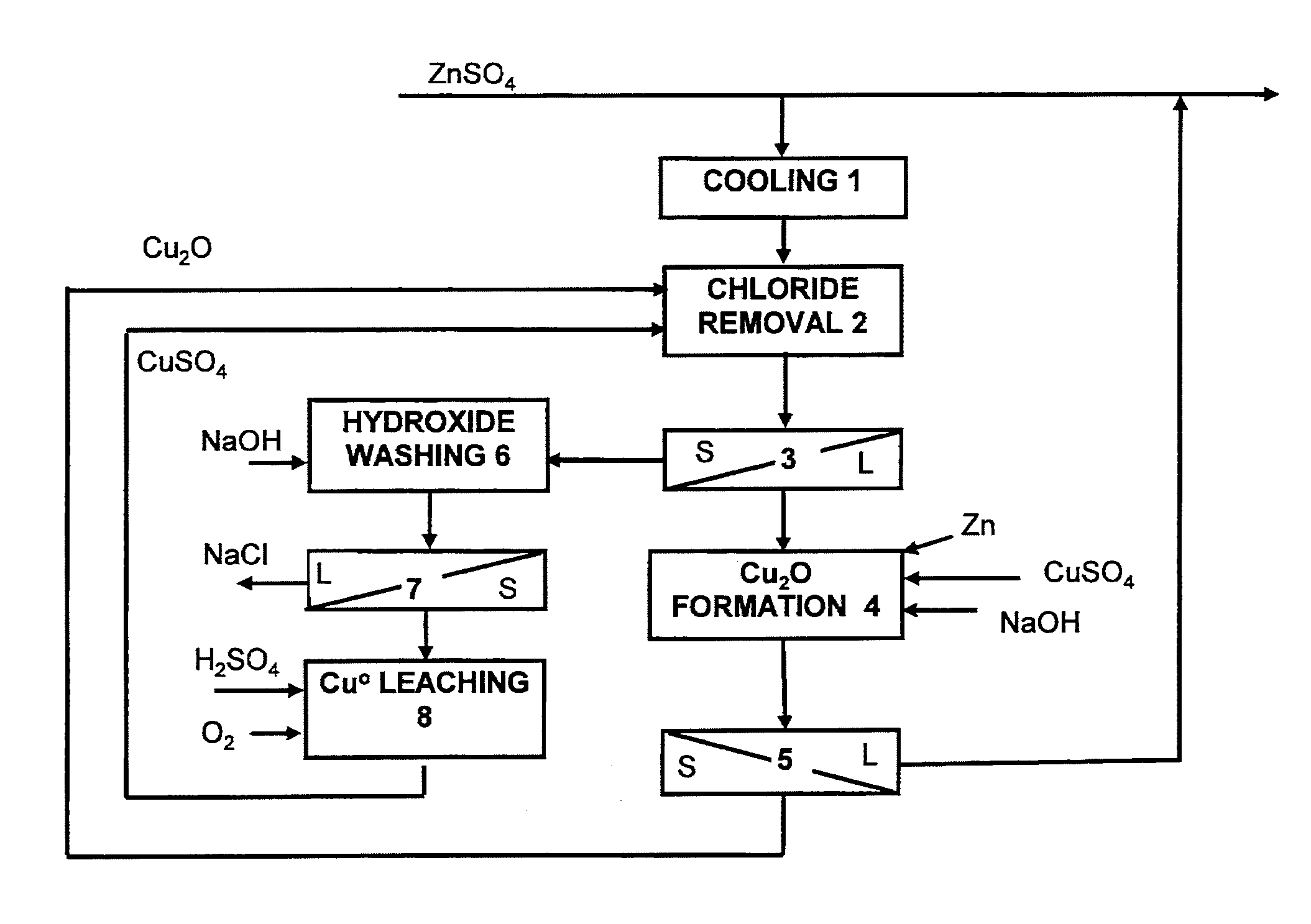
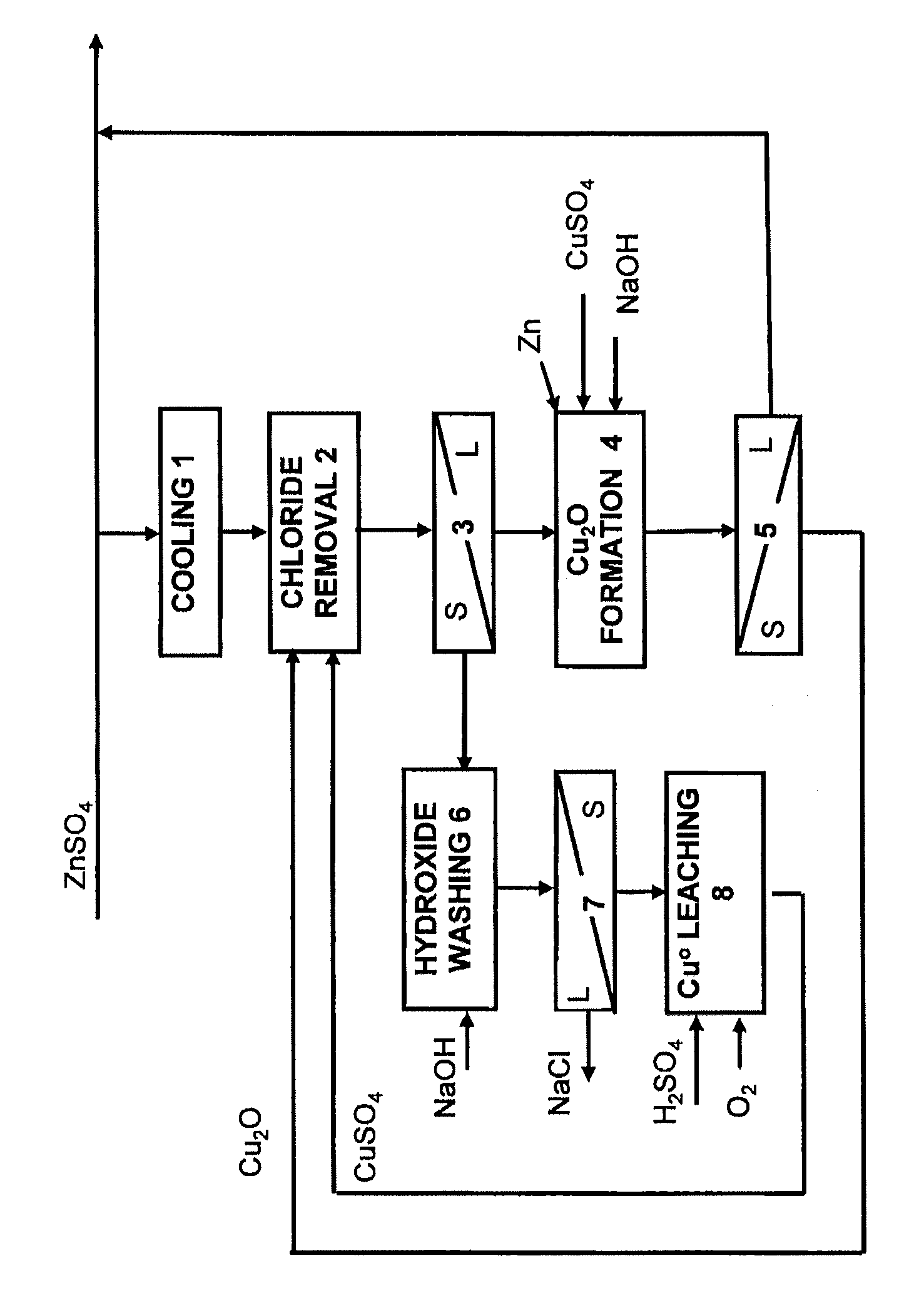
Method for the removal of chloride from zinc sulphate solution
The invention relates to a method for removing chloride from zinc sulphate solution in conjunction with zinc production. According to the method, the chloride is removed from solution by means of monovalent copper, which is produced in a separate copper(I) oxide formation stage, in which the pH is regulated to the region of 4.5-5.
Owner:METSO OUTOTEC (FINLAND) OY
Ointment for combating and treating skin diseases, and process for preparing said ointment
InactiveUS20090047359A1Simple designEasy to carrySalicyclic acid active ingredientsHeavy metal active ingredientsDiseaseWhite petrolatum
The invention relates to an ointment for treating and curing skin diseases of human beings such as psoriasis, dermatitis, acne, herpes and fungi, consisting of a composition obtained based on ingredients such as white petrolatum, clobetasol propionate, distilled water, rosemary honey, virgin olive oil, white precipitate mercury chloride, salicylic acid, and gentian violet.It also describes a process for preparing said ointment basically consisting of preparing a completely homogenized composition from the mentioned ingredients with the use of a stirring device for a time of about 30 minutes and maintaining the temperature at around 20° C. in order to obtain the cream or ointment appearance of said composition.
Owner:PRIETO JOSE RAMOS
Process for the recovery of zinc from aqueous process streams
InactiveUS6036929AReduced zinc contentReduce concentrationZinc halidesZinc oxides/hydroxidesZinc compoundsChloride
The invention provides a process for the removal and recovery of zinc from an aqueous process stream. In particular, the process of the invention is useful in the removal and recovery of zinc compounds such as zinc chloride from an aqueous effluent stream produced in various manufacturing processes such as the manufacture of sorbic acid.
Owner:EASTMAN CHEM CO
Process for recovering zinc and/or zinc oxide ii
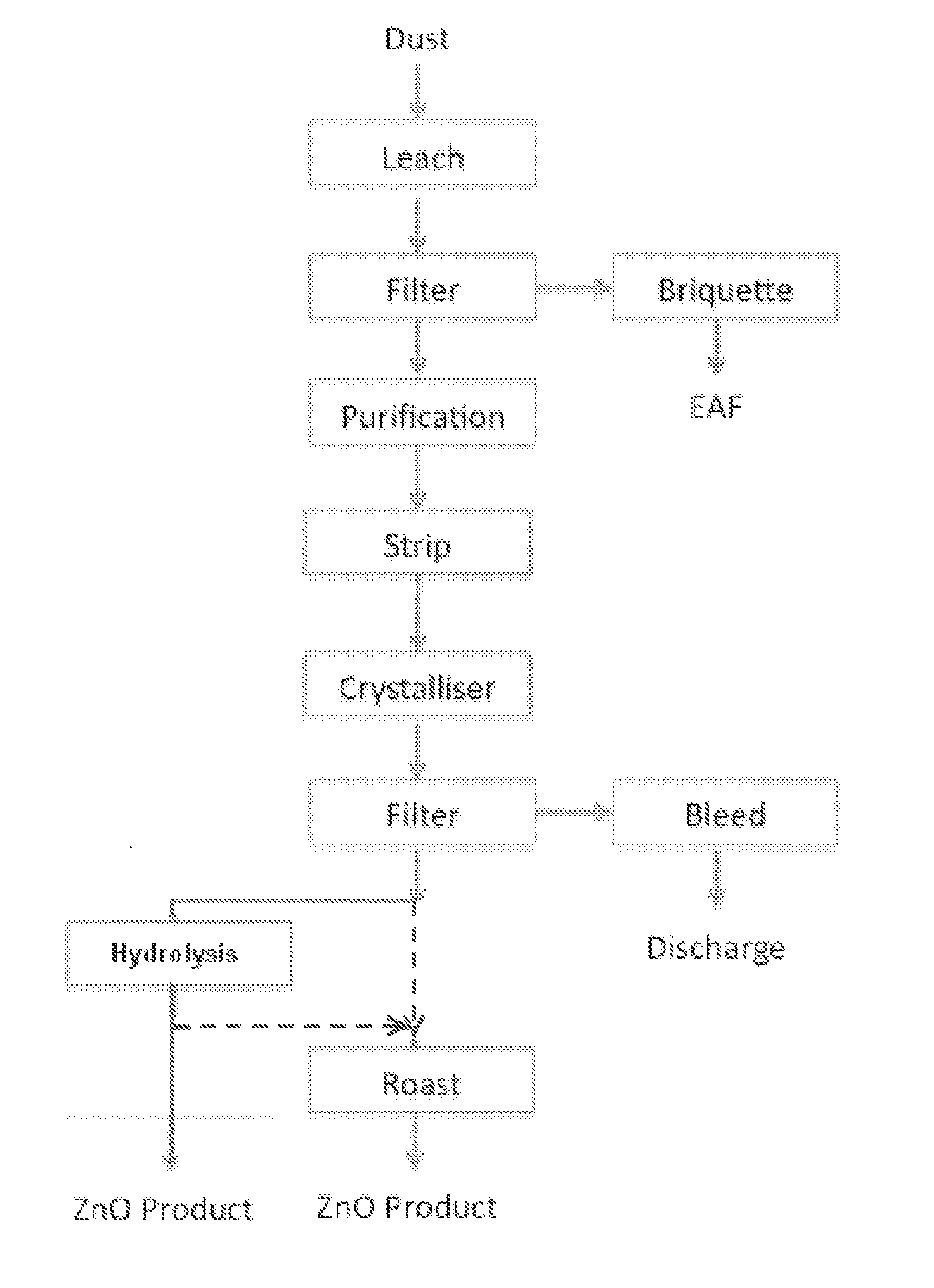
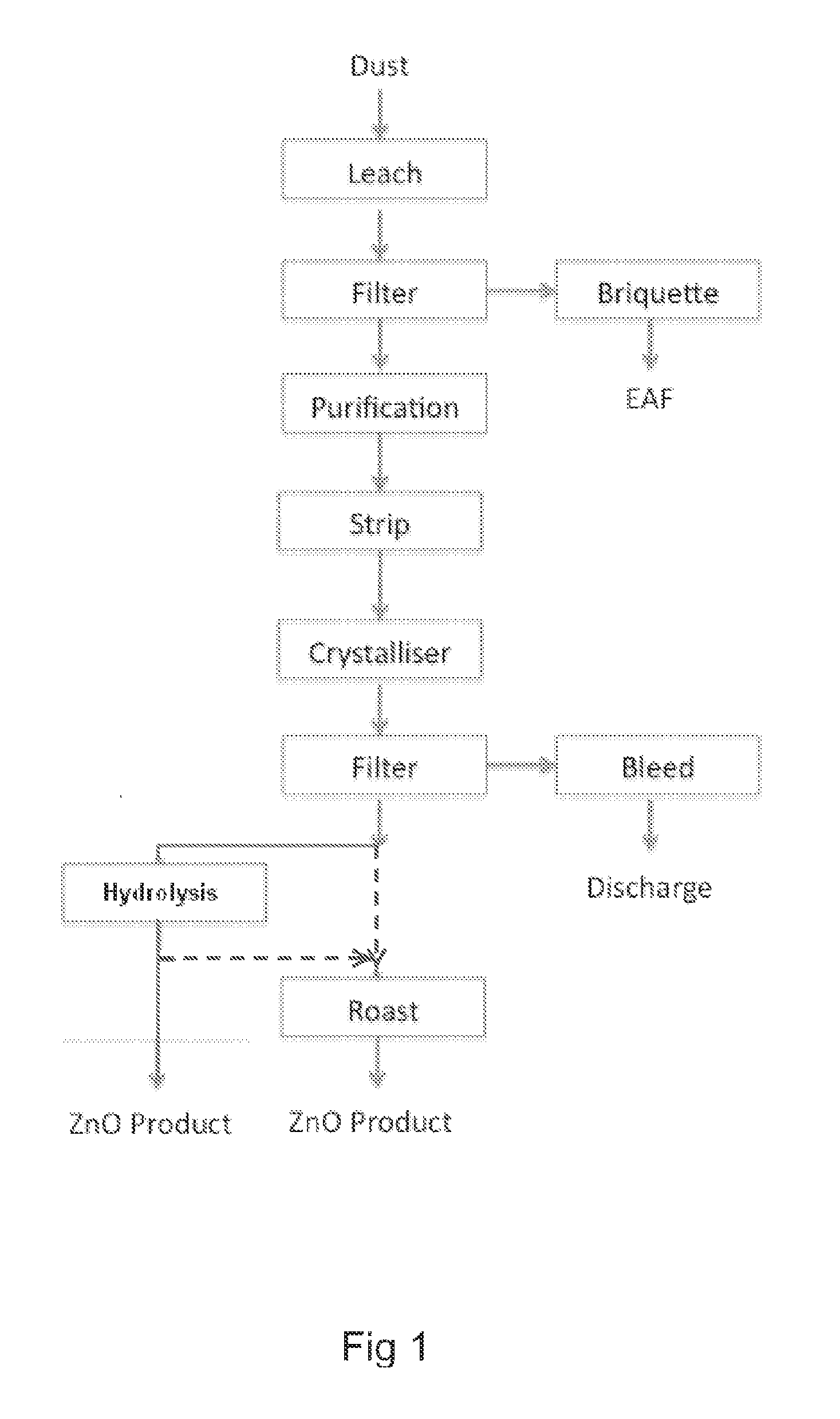
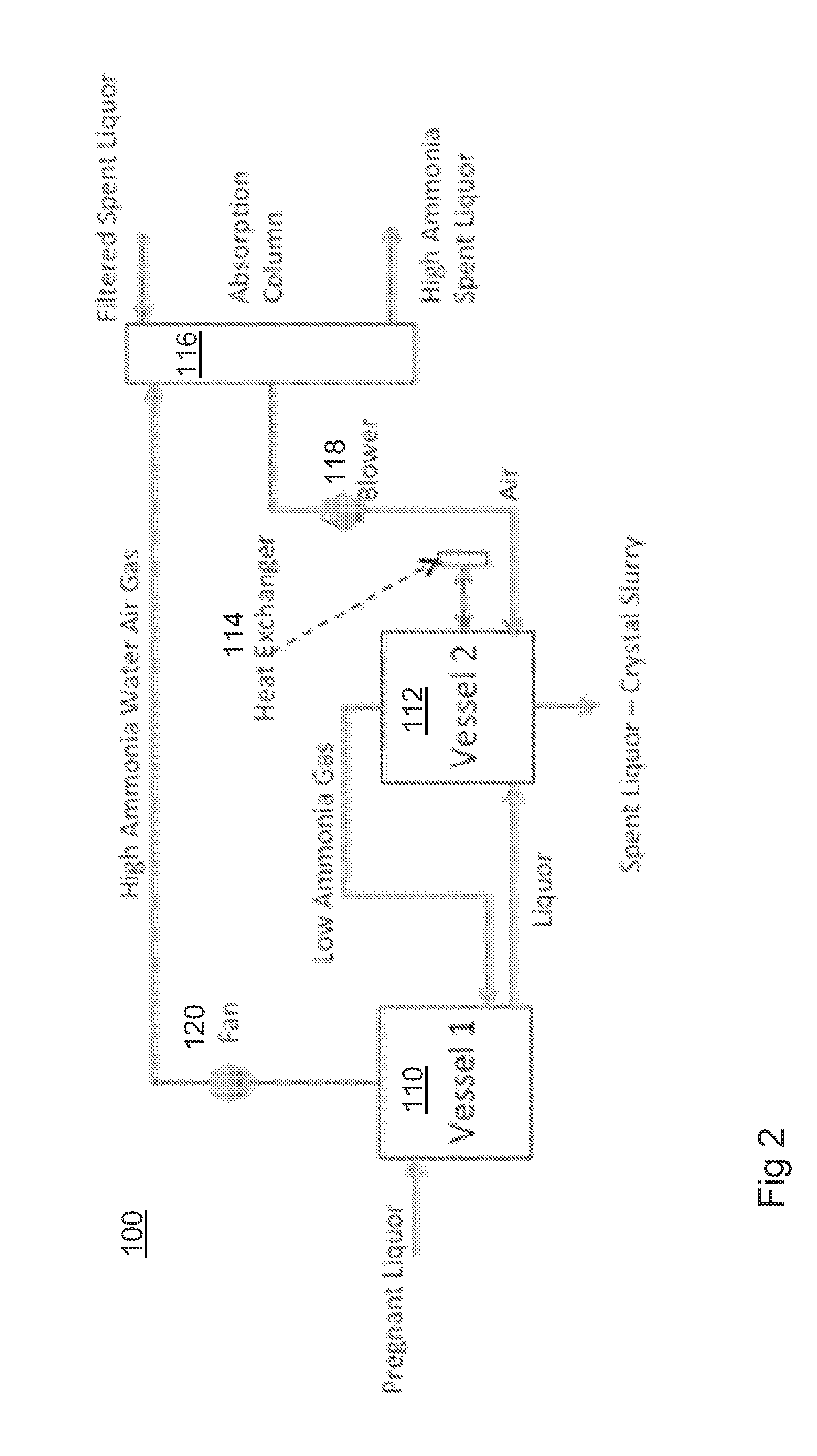
ActiveUS20140205519A1Maximum recoveryImprove featuresZinc halidesProcess efficiency improvementAmmoniaOxide zinc
A process for recovering zinc from a zinc containing material, the process including the steps of: leaching the zinc containing material with an alkaline lixiviant comprising an aqueous mixture of NH3 and NH4Cl, or ionic equivalent, having a NH4Cl concentration of between about 10 g / L and about 150 g / L H2O and a NH3 concentration of between 20 g / l H2O and 250 g / L H2O, to produce a zinc containing leachate; stripping ammonia from the leachate to produce a stripped liquor which includes a zinc containing precipitate, the stripped liquor having a NH3 concentration of between 7 and 30 g / L H2O; and recovering the zinc from the stripped liquor.
Owner:STEEL DYNAMICS INVESTMENTS LLC
Metal Recovery Process
ActiveUS20120114539A1Reduce the temperatureLess energyZinc halidesChloride preparationMetal chlorideZinc smelting
A process for recovering a metal chloride or mixed metal chloride from a solid waste material comprising recoverable metal containing constituents produced by lead, copper or zinc smelting and refining processes, said process comprising the steps of: (i) heating the solid waste material; (ii) treating the heated material of step (i) with a gaseous chloride to form a gaseous metal chloride containing product; and (iii) treating the gaseous metal chloride containing product of step (ii) to recover the metal chloride or mixed metal chloride. The metal chloride may be further treated to extract the metal itself.
Owner:MINEX TECH
Method for recycling waste mercury catalyst from polyvinyl chloride production based on calcium carbide method
ActiveCN106673055AHigh purityEfficient removalCarbon compoundsOther chemical processesActivated carbonPolyvinyl chloride
The invention provides a method for recycling a waste mercury catalyst from polyvinyl chloride production based on a calcium carbide method. The method comprises the following steps: smashing the waste mercury catalyst with mercuric chloride, hydrogen chloride and vinyl chloride attached to activated carbon into certain fineness; extracting with carbon disulfide and filtering, thereby acquiring filter residues; heating and then condensing the filter residues under a light-shielding condition to recycle mercuric chloride; treating the filter residues with alkali, neutralizing the absorbed hydrogen chloride, filtering, drying and recycling the activated carbon. The recycling method provided by the invention has the advantages that the heating temperature for recycling the mercuric chloride is low, the recycling of the mercuric chloride can be carried out only at 90-120 DEG C, the recovery rate reaches up to 95% and the purity reaches up to 99.5%; after the recycled activated carbon is treated, the purity is high, the recovery rate reaches up to 92%, the absorption activity is recovered, the micro-pore surface area is recovered to 1000-1200m<2> / g and the pore volume is recovered to 0.3-0.6ml / g; besides, according to the method, the operation is simple, the energy consumption is low, the environment-friendly and pollution-free effects are achieved; the organic substances, such as chloroethylene, and the hydrogen chloride acidic material can be effectively removed.
Owner:HWASU
Method for preparing lead chloride and calomel from mercury-containing waste catalyst
The invention provides a method for preparing lead chloride and calomel from a mercury-containing waste catalyst and relates to the technical field of three waste treatment and utilization of organic industrial mercury-containing waste catalysts. The mercury-containing waste catalyst is treated to produce lead chloride and calomel by the following three steps: firstly the mercury-containing waste catalyst is fed to a closed-type distiller to be distilled so that lead chloride is directly sublimated to a gas, and then the gas is cooled to produce liquid lead chloride; secondly the resulting lead chloride liquid reacts with lead particles to produce a mixed liquid of lead chloride and calomel, the mixed liquid is filtered under sealed conditions to produce a filter cake of lead chloride, and the filter cake of lead chloride is washed, dehydrated and packaged to obtain a lead chloride product; and finally the resulting calomel liquid is fed to a closed-type normal-pressure distiller to be distilled so that calomel is directly sublimated, and the sublimate is cooled and crystallized to obtain a calomel crystal product. The method effectively avoids the environmental pollution due to the mercury-containing waste catalyst and simultaneously creates higher economic benefits.
Owner:何侠
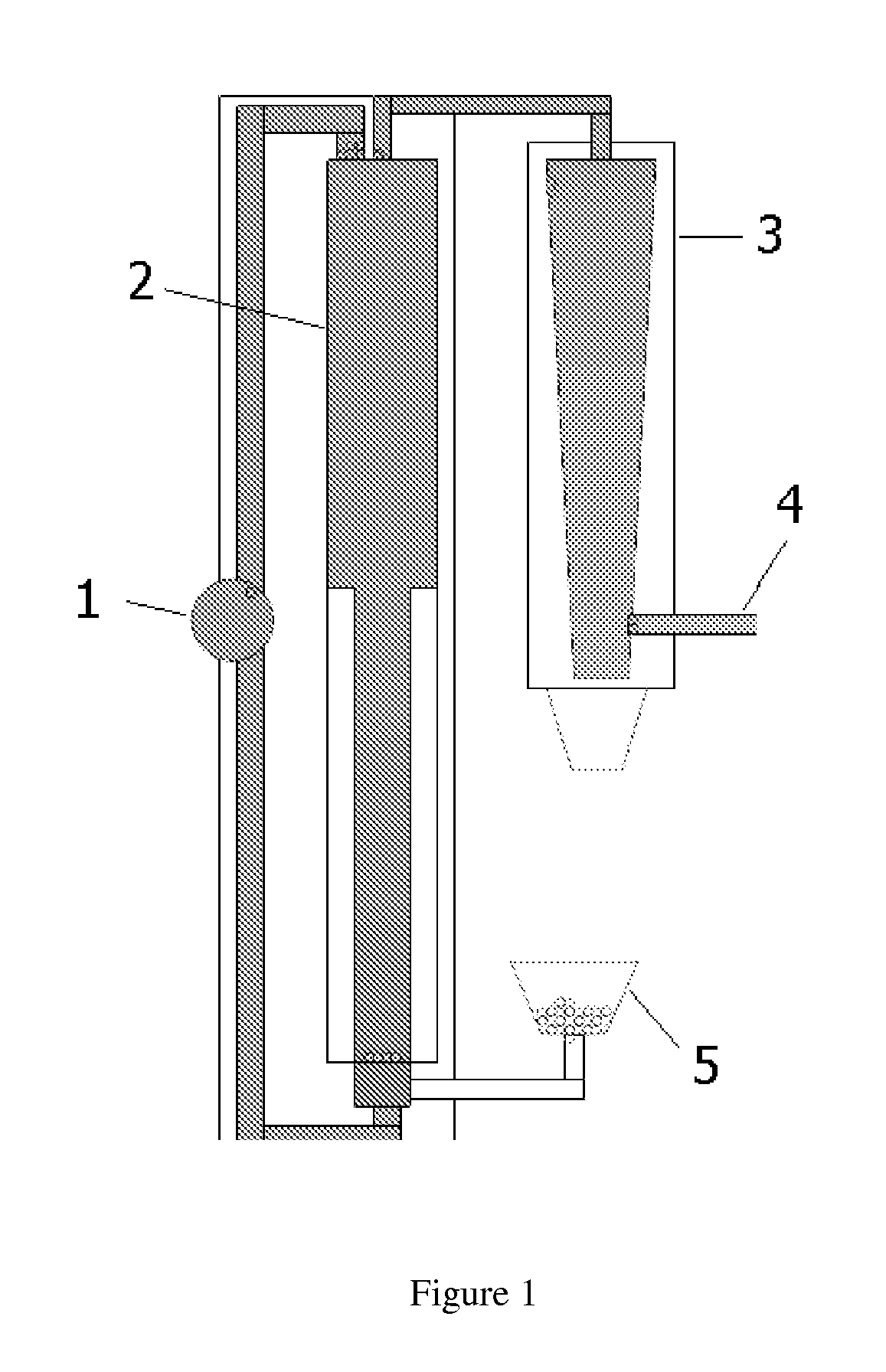
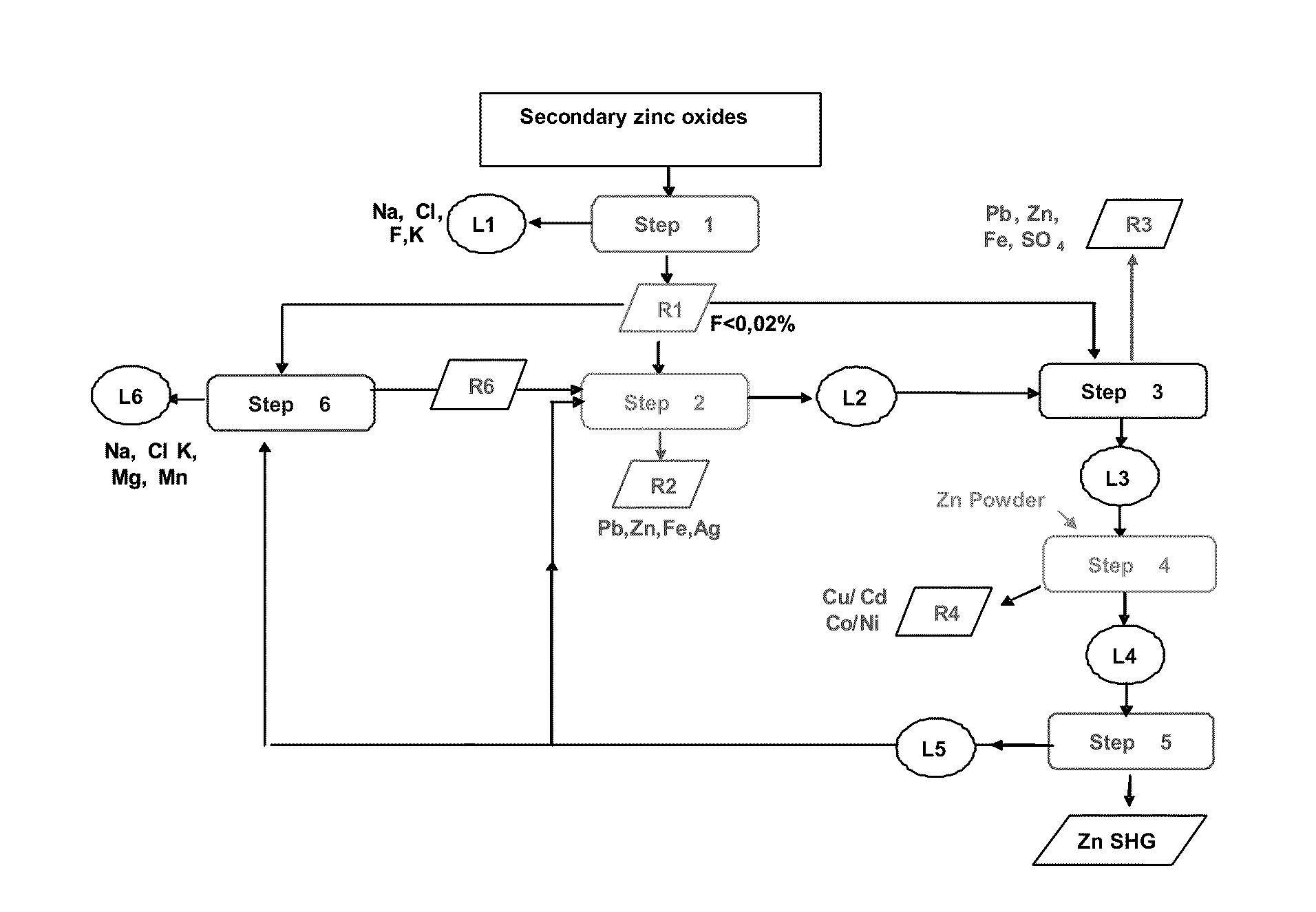
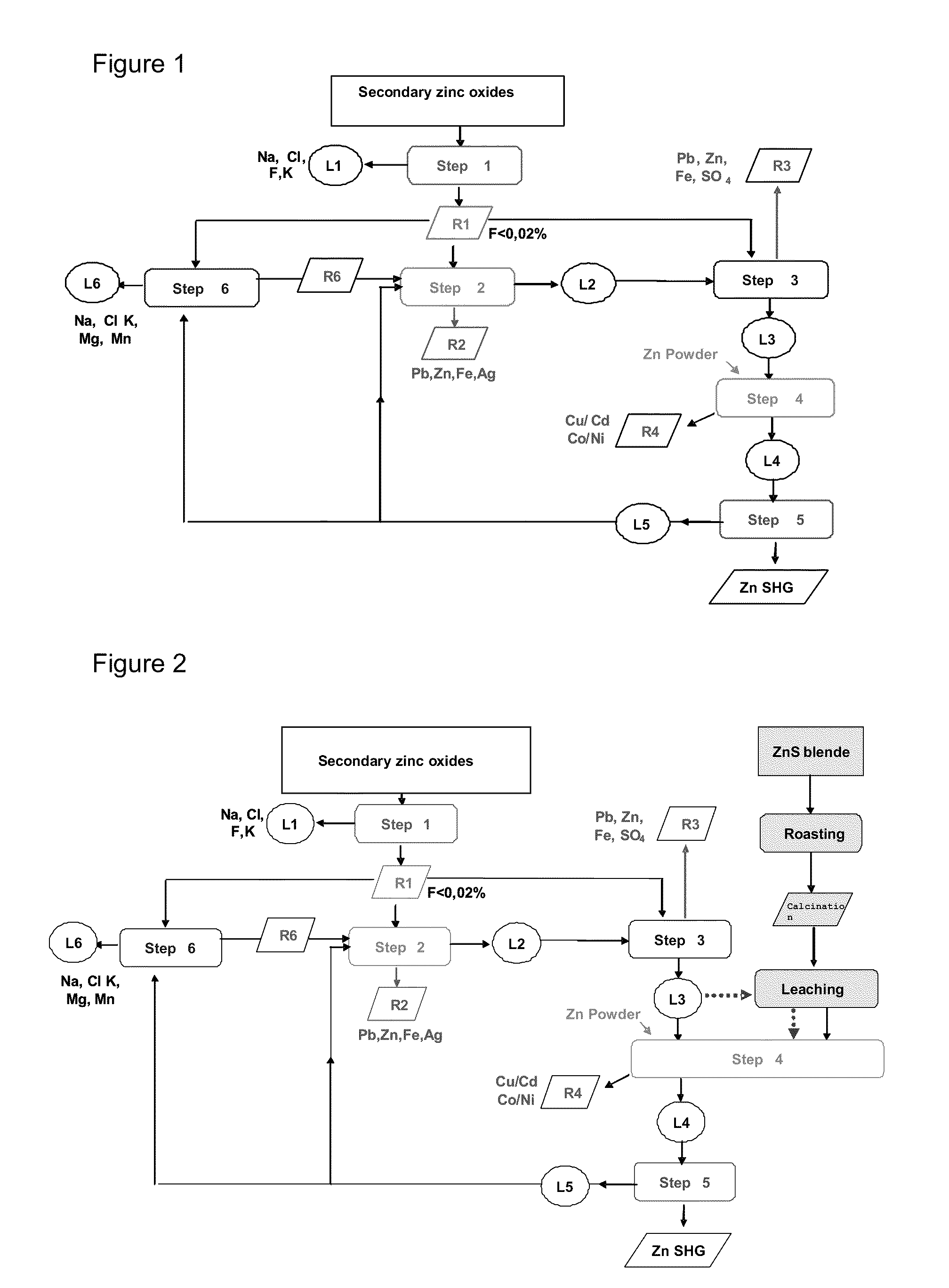
Hydrometallurgical method for the reuse of secondary zinc oxides rich in fluoride and chloride
InactiveUS20110268632A1Reduce consumptionReduce operating costsZinc halidesPhotography auxillary processesChlorideZinc
The present invention relates to a method for removing halides, in particular chlorides and fluorides, from starting secondary zinc oxides, for example Waelz or Primus oxides, comprising the steps (1) for washing the secondary zinc oxides with sodium carbonate and separating the solid residue from the basic liquid, (2) leaching at least one portion of the solid residue of step 1 by means of H2SO4, preferably up to a pH between 2.5 and 4, and separating the solid residue from the acid liquid, and (3) treating the liquid from step 2 by adding Al3+ and PO43− ions and a neutralizing agent in order to remove the residual fluoride, preferably at a pH<4, and separating the liquid from the solid residue containing fluorides.
Owner:PAUL WURTH SA
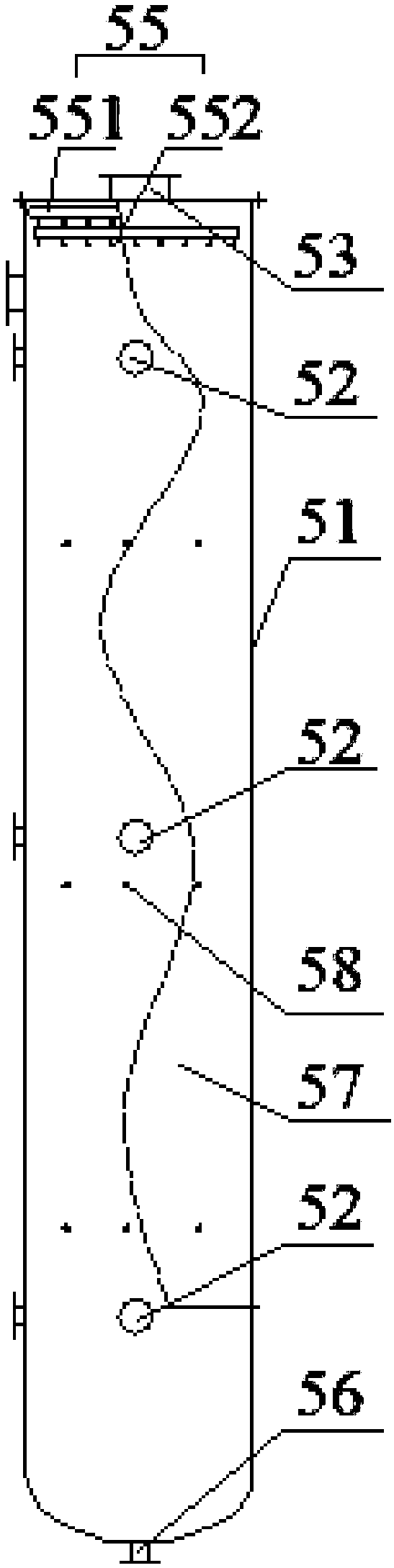
Efficient recovery process and device for waste mercury catalyst
ActiveCN108545741AHigh recovery rateLow recovery rateCarbon compoundsChemical recyclingActivated carbonInternal temperature
The invention provides an efficient recovery process for a waste mercury catalyst. The process is characterized in that the waste mercury catalyst to be heated is added to a recovery furnace, and therecovery furnace is heated until the internal temperature of the furnace at least reaches 320 DEG C to enable mercury chloride in the waste mercury catalyst to be separated from activated carbon in agaseous form to obtain high temperature gas containing mercury chloride and simultaneously obtain recyclable activated carbon; and water with the temperature not higher than 70 DEG C is adopted to acton the high temperature gas containing mercury chloride, after the high temperature gas containing mercury chloride is in contact with the water, mercury chloride in the high temperature gas is dissolved in the water to obtain recyclable mercury chloride liquid, and the recovered mercury chloride liquid can be repeatedly used for spraying the high temperature gas containing mercury chloride. Theefficient recovery process and device provided by the invention can efficiently recover activated carbon and almost recover all mercury chloride and are efficient and pollution-free.
Owner:张良儒
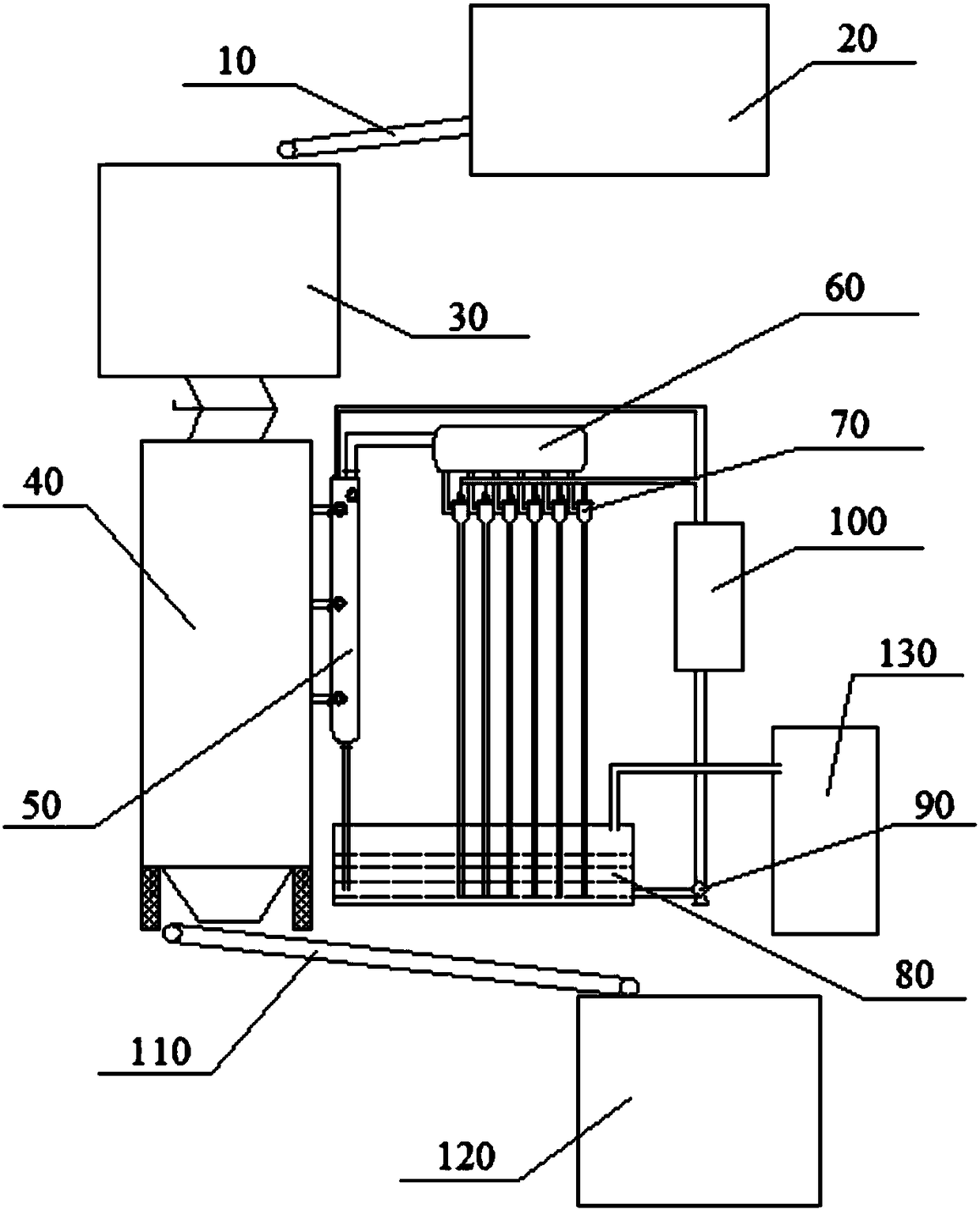
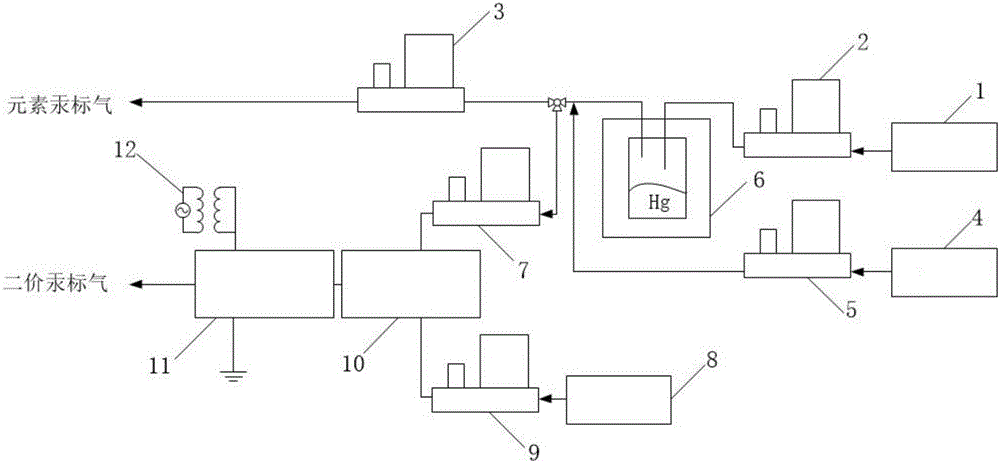
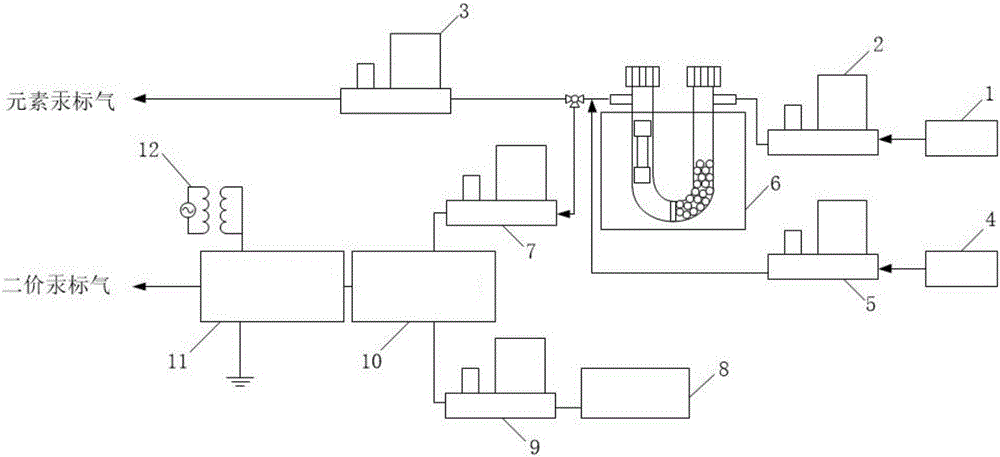
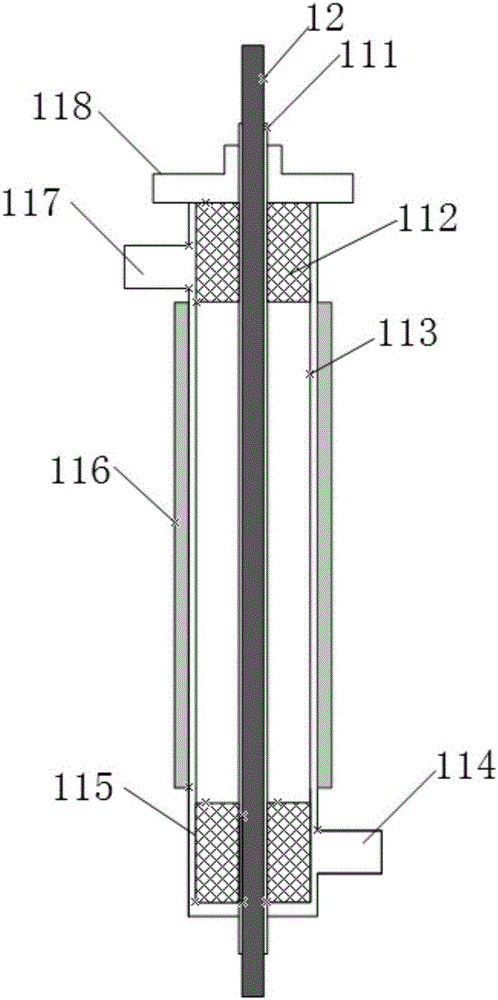
Device for producing mercury standard gas and divalent mercury standard gas on basis of saturation principle
ActiveCN105886796AAvoid the problem that inconsistencies require two traceability passesEasy to operateMercury oxidesMercury halidesAlternating currentHigh pressure
The invention discloses a device for producing mercury standard gas and divalent mercury standard gas on the basis of the saturation principle. The device comprises an element mercury standard gas generating system and a divalent mercury standard gas generating system, wherein the element mercury standard gas generating system comprises a first carrier gas device and an element mercury generator which are sequentially connected by the aid of a pipeline, and the element mercury generator is used for producing mercury gas; the divalent mercury standard gas generating system comprises a plasma gas source device, a gas premixing chamber and a divalent mercury generator which are sequentially connected by the aid of a pipeline, the divalent mercury generator is used for producing divalent mercury gas, the gas premixing chamber is further connected with an outlet of the element mercury generator and is used for mixing a plasma gas source with mercury-containing gas produced by the element mercury generator, the divalent mercury generator is provided with a high-frequency high-voltage alternating-current power generator and is used for producing plasma to oxidize the mercury-containing gas into divalent mercury gas, the element mercury standard gas and the divalent mercury standard gas are obtained simultaneously, the same mercury source is adopted, the problem that source tracing delivery is required to be performed twice due to inconsistent mercury sources caused by adoption of the different mercury sources is solved, and operation steps are simplified.
Owner:CHINA HUADIAN SCI & TECH INST
Device for producing mercury and bivalent mercury standard gas based on penetration principle
InactiveCN105699146AAvoid the problem that inconsistencies require two traceability passesEasy to operatePreparing sample for investigationMercury oxidesHigh pressurePlasma Gases
The invention discloses a device for producing mercury and bivalent mercury standard gas based on the penetration principle. The device comprises an element mercury standard gas generating system and a bivalent mercury standard gas generating system. The element mercury standard gas generating system comprises a first gas carrier device and an element mercury generator for generating mercury gas, wherein the first gas carrier device and the element mercury generator are sequentially connected through a pipeline. The bivalent mercury standard gas generating system comprises a plasma gas source device, a gas premixing chamber and a bivalent mercury generator which are sequentially connected through a pipeline, wherein the bivalent mercury generator produces bivalent mercury gas. The gas premixing chamber is further connected with an outlet of the element mercury generator and used for mixing a plasma gas source and mercury gas produced by the element mercury generator, the bivalent mercury generator is provided with a high-frequency high-voltage alternating current power source generation device and used for generating plasmas to oxidize mercury gas into bivalent mercury gas, element mercury standard gas and bivalent mercury standard gas are obtained at the same time, one mercury source is adopted, the problem that traceability delivery needs to be carried out two times when different mercury sources are adopted is avoided, and operation steps are simplified.
Owner:CHINA HUADIAN SCI & TECH INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com