New medical application of baicalein and its prodrugs
The technology of a prodrug, baicalein, applied in the field of medicine, can solve the problem of not finding the application of baicalein, and achieve the effects of good prospects, economic benefits, controllable costs, and convenient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
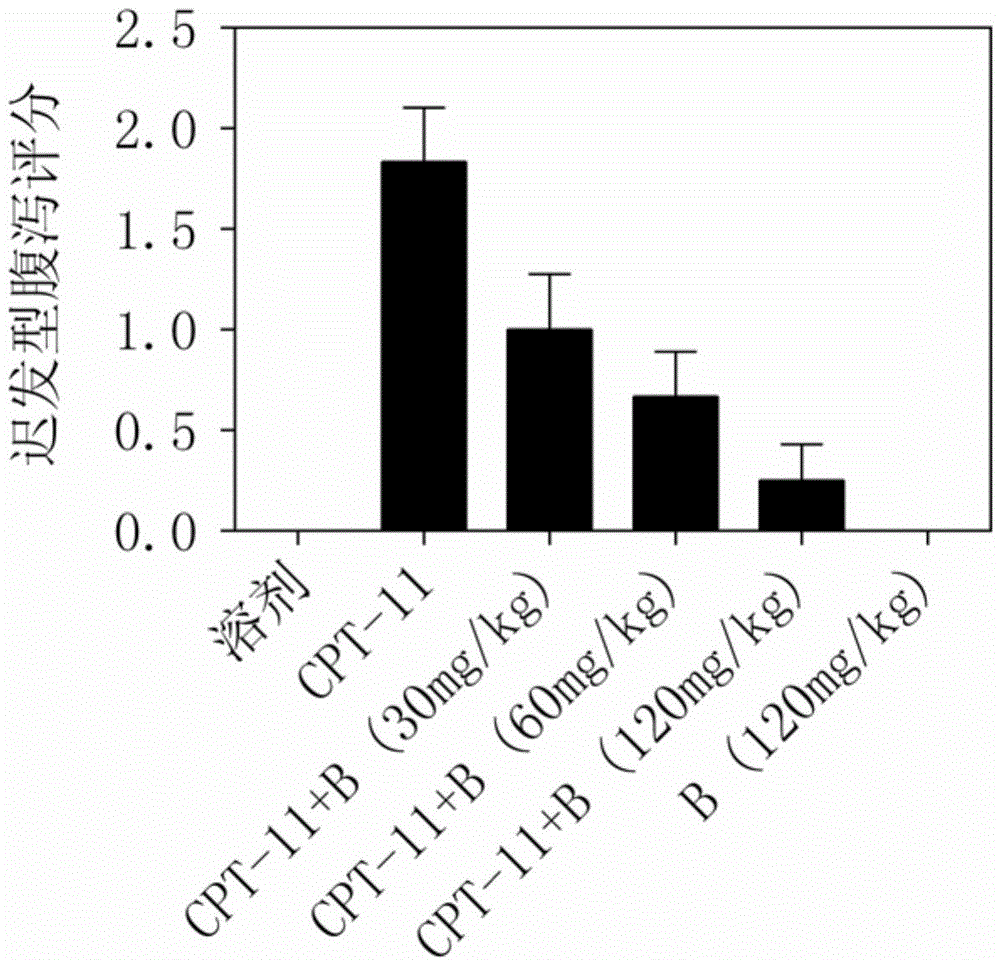
[0032] Baicalein or baicalein triethyl ester relieves irinotecan-induced diarrhea model experiment: 36 Balb / c mice were randomly divided into 6 groups: solvent control group, irinotecan model group, irinotecan + baicalein or baicalein Triethyl ester (30, 60, 120 mg / kg) group, baicalein or baicalein triethyl ester group, 6 rats in each group. The diarrhea model adopts intraperitoneal injection of irinotecan (100mg / kg / d) for 3 consecutive days. The delayed diarrhea appears on the 4th day and becomes the most serious on the 5th day. One day before irinotecan injection, baicalein or baicalein triethyl ester was given by intragastric administration (twice a day). The diarrhea status of the mice in each group was observed separately, and the mice were killed on the 6th day, and the intestinal tissue was collected, and the tissue section was stained for examination.
[0033] (1) Evaluation of diarrhea in mice
[0034] Record the diarrhea situation of the mice in each group on the 4...
Embodiment 2
[0044] Baicalein glucuronide conjugate relieves the diarrhea model experiment caused by cisplatin: 36 Balb / c mice were randomly divided into 6 groups: solvent control group, cisplatin model group, cisplatin + baicalein glucuronide conjugate (40 , 80, 120mg / kg) group, baicalein glucuronide group, 6 rats in each group. Diarrhea model adopts intraperitoneal injection of cisplatin (10mg / kg / d) for 3 consecutive days. Delayed diarrhea appears on the 4th day and is most severe on the 5th day. One day before the injection of cisplatin, baicalein glucuronide was given by intragastric administration (twice a day). The diarrhea status of the mice in each group was observed separately, and the mice were killed on the 6th day, and the intestinal tissue was collected, and the tissue section was stained for inspection.
[0045] (1) Evaluation of diarrhea in mice
[0046] Record the diarrhea situation of the mice in each group on the 4th and 5th day after the injection of cisplatin, observe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com