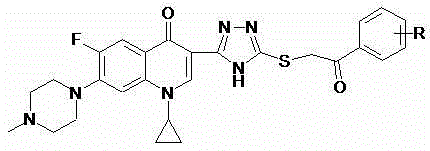
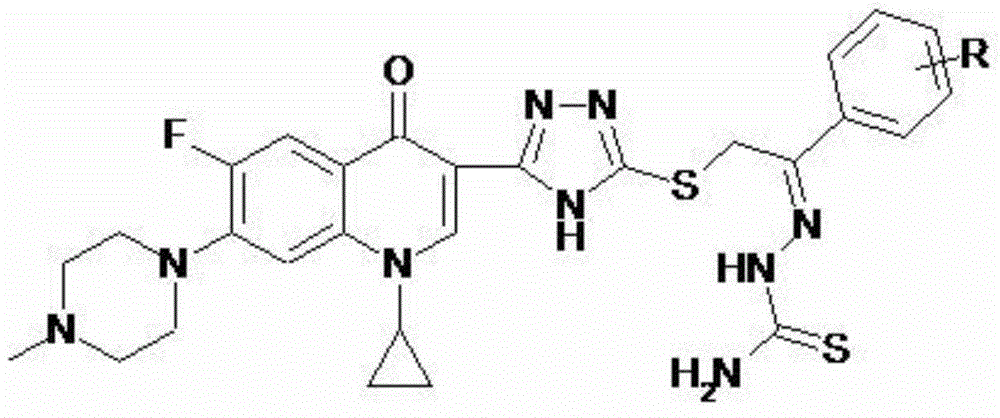
Cyclopropylfluoroquinolone C-3 s-triazole thioether ketone thiosemicarbazone compound and preparation method and application thereof
A technology of azole thioetherketone thiosemicarbazone and cyprofluoroquinolone, which is applied in the field of drug synthesis and can solve the problems of low tumor therapeutic index and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
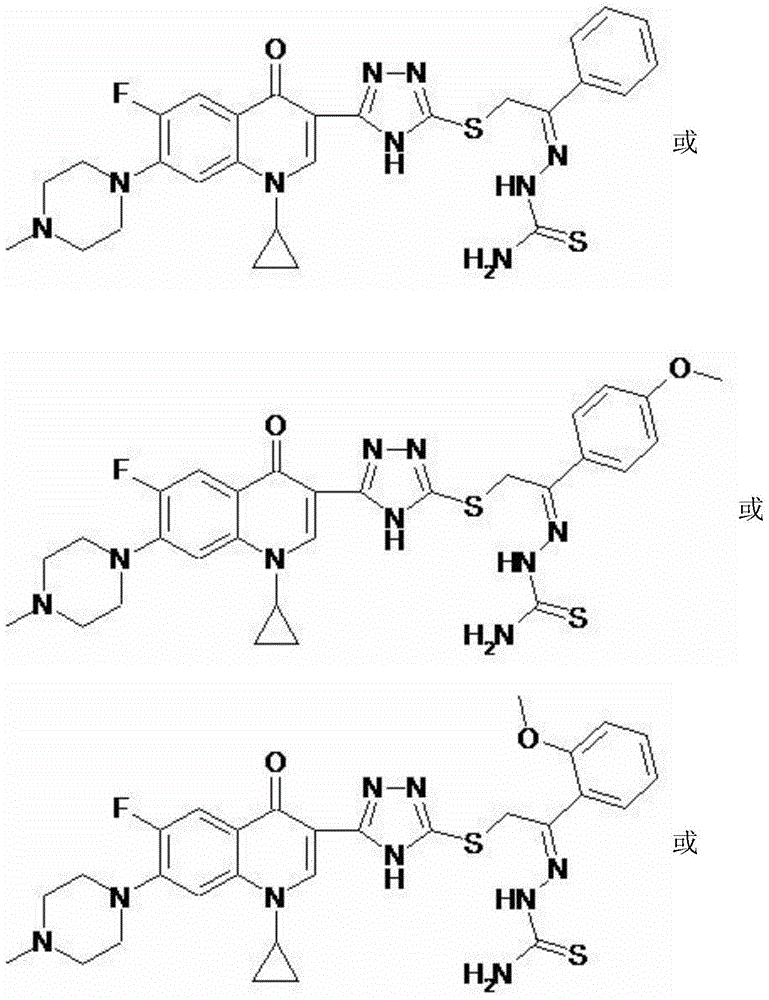
[0046] 2-{5-[1-Cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-4H-[1 ,2,4] Triazole-3-thio group}-1-acetophenone thiosemicarbazide (I-1), its chemical structural formula is:
[0047]
[0048] That is, R in formula I is a hydrogen atom.
[0049] The preparation method of the compound is as follows: taking the intermediate (S)-2-{5-[1-cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4( 1H)-Keto-3-yl]-4H-[1,2,4]triazole-3-sulfanyl}-1-acetophenone (VI-1) (2.0 g, 3.9 mmol) was dissolved in glacial acetic acid ( 20 mL), thiosemicarbazide (0.42 g, 4.7 mmol) was added, and the mixture was refluxed for 6 h. The solvent was evaporated under reduced pressure, water (30 mL) was added to dissolve, 0.1 g of activated carbon was added, and the mixture was stirred and decolorized at 60° C. for 1 h. The filtrate was adjusted to neutrality with concentrated ammonia water, and the resulting solid was collected by filtration, washed with water, dried, and r...
Embodiment 2
[0051] 2-{5-[1-Cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-4H-[1 ,2,4]Triazole-3-thio}-1-(p-methoxyphenyl)-acetone thiosemicarbazide (I-2), its chemical structural formula is:
[0052]
[0053] That is, R in formula I is p-methoxy.
[0054]The preparation method of the compound is as follows: taking the intermediate (S)-2-{5-[1-cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4( 1H)-keto-3-yl]-4H-[1,2,4]triazole-3-sulfanyl}-1-(p-methoxyphenyl)-ethanone (VI-2) (2.0 g, 3.6 mmol) was dissolved in glacial acetic acid (20 mL), thiosemicarbazide (0.37 g, 4.0 mmol) was added, and the mixture was refluxed for 10 h. The solvent was evaporated under reduced pressure, water (30 mL) was added to dissolve, 0.1 g of activated carbon was added, and the mixture was stirred and decolorized at 60° C. for 1 h. The filtrate was adjusted to neutrality with concentrated ammonia water, and the resulting solid was collected by filtration, washed with water,...
Embodiment 3
[0056] 2-{5-[1-Cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-4H-[1 ,2,4]Triazole-3-thio}-1-(o-methoxyphenyl)-acetone thiosemicarbazide (I-3), its chemical structural formula is:
[0057]
[0058] That is, R in formula I is o-methoxy.
[0059] The preparation method of the compound is as follows: taking the intermediate (S)-2-{5-[1-cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4( 1H)-keto-3-yl]-4H-[1,2,4]triazole-3-sulfanyl}-1-(o-methoxyphenyl)-ethanone (VI-3) (2.0g, 3.6 mmol) was dissolved in glacial acetic acid (20 mL), thiosemicarbazide (0.49 g, 5.4 mmol) was added, and the mixture was refluxed for 12 h. The solvent was evaporated under reduced pressure, water (30 mL) was added to dissolve, 0.1 g of activated carbon was added, and the mixture was stirred and decolorized at 60° C. for 1 h. The filtrate was adjusted to neutrality with concentrated ammonia water, and the resulting solid was collected by filtration, washed with water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com