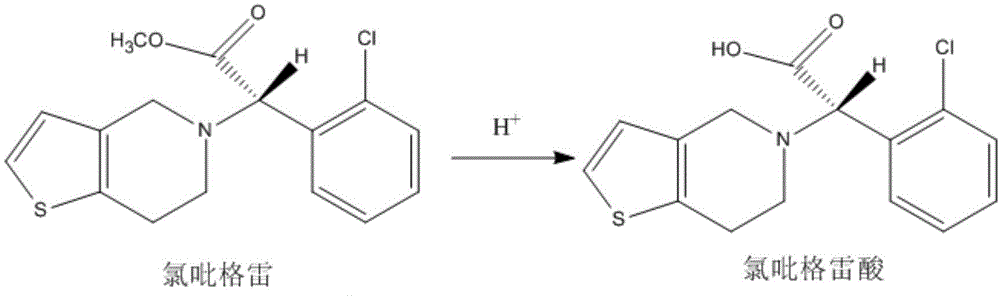
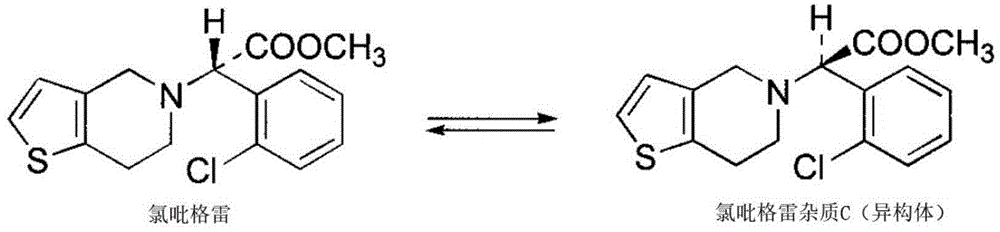
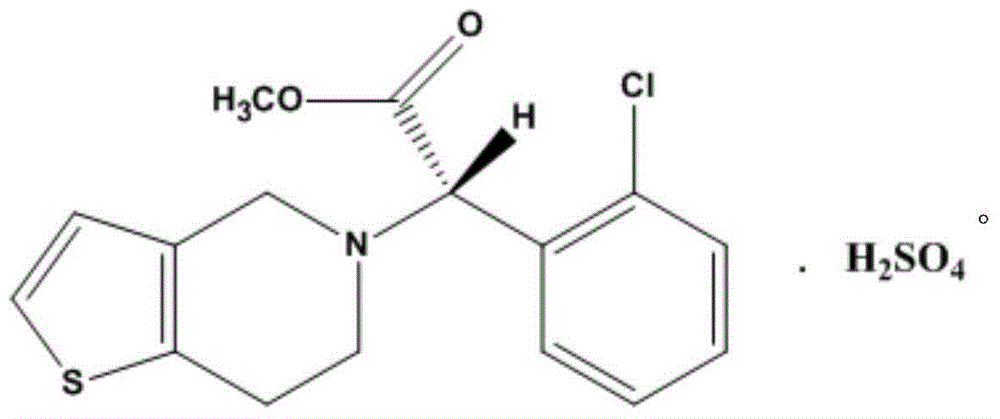
Solid pharmaceutical composition with clopidogrel
A technology of clopidogrel and its composition, which is applied in the field of solid pharmaceutical composition containing clopidogrel and its preparation, and can solve the problems of inability to wet granulation, sticky punching, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 125
[0101] Embodiment 125mg specification clopidogrel tablet preparation
[0102] Composition
Dosage (unit: g)
Clopidogrel hydrochloride (calculated as clopidogrel)
25
70
Crospovidone
6
12
2.4
Coating agent
Appropriate amount
Appropriate amount
Approx.
120mg
Co-made
1000 pieces
[0103] The preparation process is as follows:
[0104] 1) Use a dehumidifier to dehumidify the operating space, and control the ambient humidity to less than 20%;
[0105] 2) Take the clopidogrel bulk drug, pulverize it so that the particle size is less than 25 microns, and set aside;
[0106] 3) Take the prescription amount and pulverize the clopidogrel raw material drug, mannitol, and L-glutamic acid, mix evenly, dry granulate, and granulate with a 24-mesh sieve to obtain dry granules containing clopidogrel;
[0107] 4) Take the...
Embodiment 275
[0112] Embodiment 275mg specification clopidogrel tablet preparation
[0113] Composition
Dosage (unit: g)
Clopidogrel hydrochloride (calculated as clopidogrel)
75
135
Crospovidone
13.5
27
5.4
Coating agent
Appropriate amount
Appropriate amount
Approx.
270
Co-made
1000 pieces
[0114] The preparation process is as follows:
[0115] 1) Use a dehumidifier to dehumidify the operating space, and control the ambient humidity to less than 20%;
[0116] 2) Take the clopidogrel bulk drug, pulverize it so that the particle size is less than 25 microns, and set aside;
[0117] 3) Take the prescription amount and pulverize the clopidogrel raw material drug, mannitol, and L-glutamic acid, mix evenly, dry granulate, and granulate with a 24-mesh sieve to obtain dry granules containing clopidogrel;
[0118] 4) Take t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com