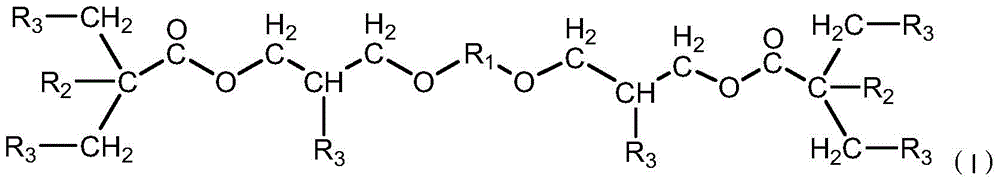
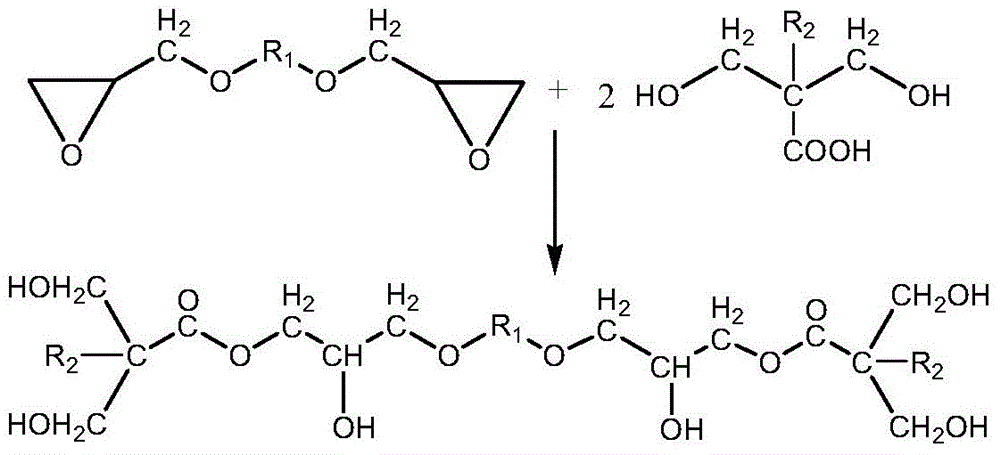
A kind of polyfunctional polyurethane acrylate and its preparation method and application
A technology of urethane acrylate and hydroxy acrylate, which is applied in the field of multifunctional urethane acrylate and its synthesis, can solve problems such as decreased adhesion, poor hardness, and increased brittleness of the coating film, achieving enhanced adhesion, less side reactions, The effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Synthesis of semi-adducts
[0055] Under the protection of nitrogen, 116.12 grams of hydroxyethyl acrylate and 1.0451 grams of dibutyltin dilaurate were added dropwise to 233.4045 grams of isophorone diisocyanate, and the reaction temperature was maintained at 40 ° C for 2 hours to obtain ‐NCO semi-blocked semi Adduct (A). Take a sample to check the concentration of ‐NCO groups in the system to determine if the reaction is complete.
[0056] (2) Modification of glycidyl ether
[0057] Add 128.17 grams of 1,4-cyclohexanedimethanol glycidyl ether, 134.13 grams of dimethylol propionic acid and 0.6558 grams of catalyst triphenylphosphine into the reactor to raise the temperature to form a homogeneous phase, and control the temperature at 140 ° C for 2.5 h, stop the reaction when the acid value drops below 3% of the initial acid value of the system, and react to obtain a modified glycidyl ether (B) containing 6 hydroxyl groups in the molecular structure. Take a sample...
Embodiment 2
[0063] (1) Synthesis of semi-adducts
[0064] Under the protection of nitrogen, 130.14 grams of hydroxypropyl acrylate and 1.4315 grams of dibutyltin dilaurate were added dropwise to 174.08 grams of toluene diisocyanate TDI, and the reaction temperature was maintained at 50 ° C for 1 hour to obtain the semi-addition of ‐NCO semi-blocking Object (A). Take a sample to check the concentration of ‐NCO groups in the system to determine if the reaction is complete.
[0065] (2) Modification of glycidyl ether
[0066] Add 101.18 grams of 1,4-butanediol diglycidyl ether, 148.16 grams of dimethylol butyric acid and 2.0934 grams of catalyst triphenylphosphine into the reactor to raise the temperature to form a homogeneous phase, and control the temperature at 130 ° C for 3.5 hours. The reaction is stopped when the acid value drops below 3% of the initial acid value of the system, and the modified glycidyl ether (B) containing 6 hydroxyl groups in the molecular structure is obtained by...
Embodiment 3
[0072] (1) Synthesis of semi-adducts
[0073] Under the protection of nitrogen, 130.14 grams of hydroxyethyl methacrylate and 1.2363 grams of dibutyltin dilaurate were added dropwise to 176.61 grams of hexamethylene diisocyanate, and the reaction temperature was kept at 45 °C for 1.5 hours to obtain ‐NCO semi-sealed Terminal half adduct (A). Take a sample to check the concentration of ‐NCO groups in the system to determine if the reaction is complete.
[0074] (2) Modification of glycidyl ether
[0075] Add 120.90 grams of 1,6 hexanediol diglycidyl ether, 134.13 grams of dimethylol propionic acid and 1.9128 grams of catalyst triphenylphosphine into the reactor and raise the temperature to form a homogeneous phase. Control the temperature at 140°C for 3.0 hours until When the acid value drops below 3% of the initial acid value of the system, the reaction is stopped, and the modified glycidyl ether (B) containing 6 hydroxyl groups in the molecular structure is obtained by the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com