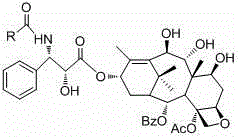
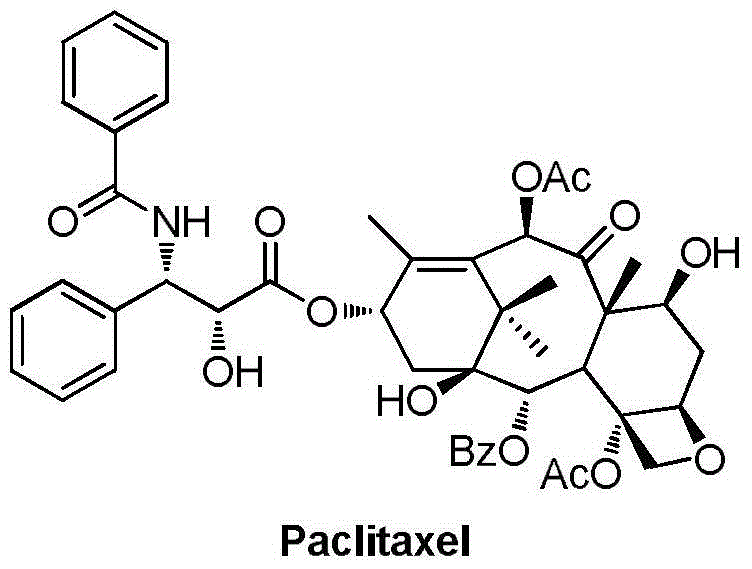
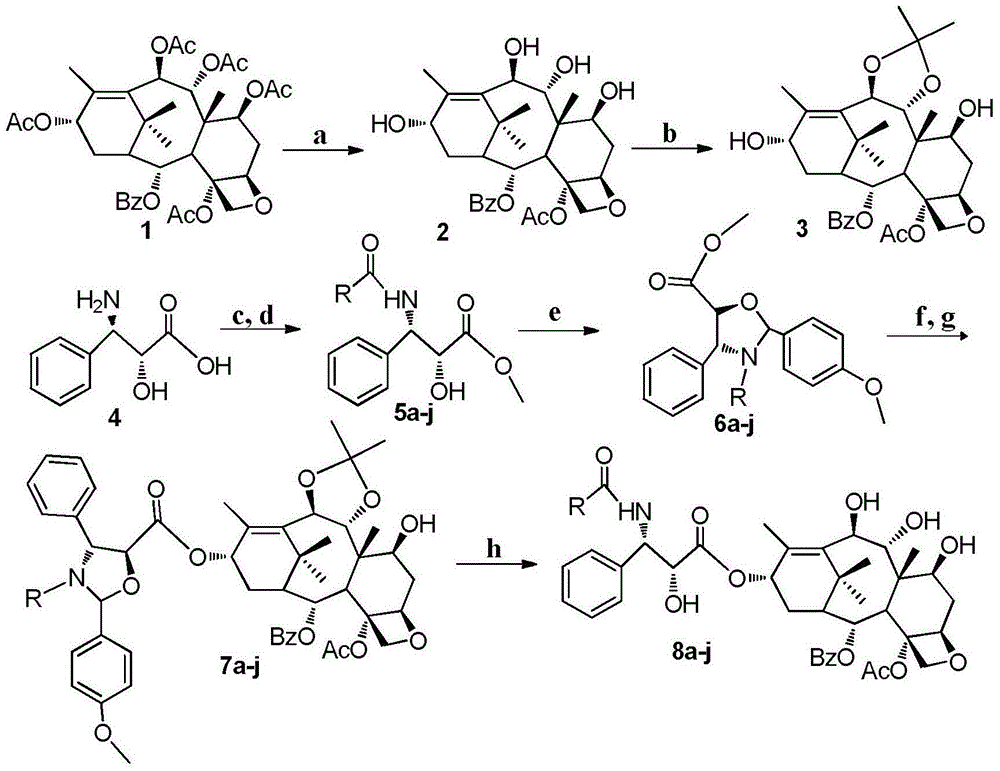
9,10-dihydroxy-1-deoxy-taxol analogue and preparation method thereof
A paclitaxel analog, dihydroxyl technology, applied in drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of limited collection, toxic and side effects, drug resistance, etc., and achieve the effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The preparation method of compound 3'-N-p-toluoyl-(7,9,10)-trideacetyl-1-dehydroxy-paclitaxel analogue, the structural formula of this compound is:
[0026]
[0027] A. 1-Deshydroxybaccatin VI1 (419 mg, 0.6 mmol) was dissolved in 20 mL of 95% ethanol, 10 mL of hydrazine hydrate was added, and stirred at room temperature for 15 hours. Neutralize with 0.2N dilute hydrochloric acid, extract with ethyl acetate, wash the organic phase three times with saturated brine, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure. The crude product was recrystallized from methanol and n-hexane to obtain 227 mg of colorless transparent crystal 7,9,10,13-tetradeacetyl-1-deoxybaccatin VI2 with a yield of 87%;
[0028] B.B. Compound 2 (239mg, 0.5mmol) was dissolved in 18mL of dichloromethane and 1.5mL of methanol, after it was completely dissolved, 2,2-dimethoxypropane (0.4mL, 2.0mmol) was added, stirred evenly, and then Mont K10 was added f...
Embodiment 2
[0038] Example 2: The preparation method of the compound 3'-N-p-methoxybenzoyl-(7,9,10)-trisdeacetyl-1-dehydroxy-paclitaxel analogue, the structural formula of the compound is:
[0039]
[0040] 8b
[0041] The method is the same as implementation example 1:
[0042] 5b: 1 HNMR (CDCl 3, 500MHz) δ(ppm): 3.85(s, 3H), 3.87(s, 3H), 4.65(s, 1H), 5.74(d, J=9.5Hz, 1H), 6.94(d, J=8.4Hz, 3H ), 7.32(t, J=15.5Hz, 1H), 7.38(t, J=15.4Hz, 2H), 7.47(d, J=8.3Hz, 2H), 7.76(d, J=8.5Hz, 2H); 13 C-NMR (125MHz, CDCl 3 )δ (ppm): 29.70, 53.29, 54.81, 55.44, 73.31, 113.84, 126.27, 126.89, 127.91, 128.54, 128.75, 128.93, 138.86, 162.71, 166.38, 173.43;
[0043] 6b: 1 HNMR (CDCl 3, 500MHz)δ(ppm):3.77(s,3H),3.82(s,6H),4.86(d,J=3.3Hz),5.47(br,1H),6.72(d,J=9.4Hz,2H), 6.87(d, J=9.3Hz, 2H), 6.94(br, 1H), 7.29-7.35(m, 6H), 7.48(br, 2H); 13 C-NMR (125MHz, CDCl 3 )δ (ppm): 14.43, 52.27, 55.29, 113.50, 127.03, 127.69, 128.01, 128.69, 128.73, 129.30, 130.08, 159.88, 161.61, 170.53;
[0044] 7b: 1 ...
Embodiment 3
[0046] Example 3: The preparation method of the compound 3'-N-p-hexylbenzoyl-(7,9,10)-trisdeacetyl-1-dehydroxy-paclitaxel analogue, the structural formula of the compound is:
[0047]
[0048] The method is the same as implementation example 1:
[0049] 5c: 1 HNMR (CDCl 3, 500MHz) δ (ppm): 1.26 (t, J = 15Hz, 3H), 2.70 (q, J = 39.3Hz, 2H), 3.76 (s, 3H), 4.10 (d, J = 4.4Hz, 1H), 4.63 (s,1H),5.77(d,J=9.6Hz,1H),7.20(d,J=6.5Hz,2H),7.26-7.36(m,4H),7.45(d,J=7.7Hz,2H) ,7.71(d,J=7.3Hz,2H),8.02(d,J=7.3Hz,1H); 13 C-NMR (125MHz, CDCl 3 )δ (ppm): 15.33, 28.78, 53.04, 55.11, 73.46, 126.98, 127.34, 127.79, 127.94, 128.05, 128.63, 130.30, 131.32, 148.45, 167.27, 173.42;
[0050] 6c: 1 HNMR (CDCl 3, 500MHz) δ (ppm): 1.20 (t, J = 15.5Hz, 3H), 2.62 (q, J = 24.4Hz, 2H), 3.82 (s, 3H), 3.83 (s, 3H), 4.10 (d, J =4.4Hz,1H),4.88(s,1H),5.47(br,1H),6.87(d,J=8.8Hz,2H),6.92(br,1H),7.07(d,J=9.4Hz,2H ), 7.26(d, J=8.4Hz, 3H), 7.31-7.34(m, 3H), 7.45(m, 2H); 13 C-NMR (125MHz, CDCl 3 )δ (ppm): 14....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com