Method for culturing hepatic-like cells in vitro and optimized hepatic-like cells cultured by this method
A technology of liver-like cells and in vitro culture, applied in the biological field, can solve the problem of low expression level and achieve the effect of improving expression level and increasing expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
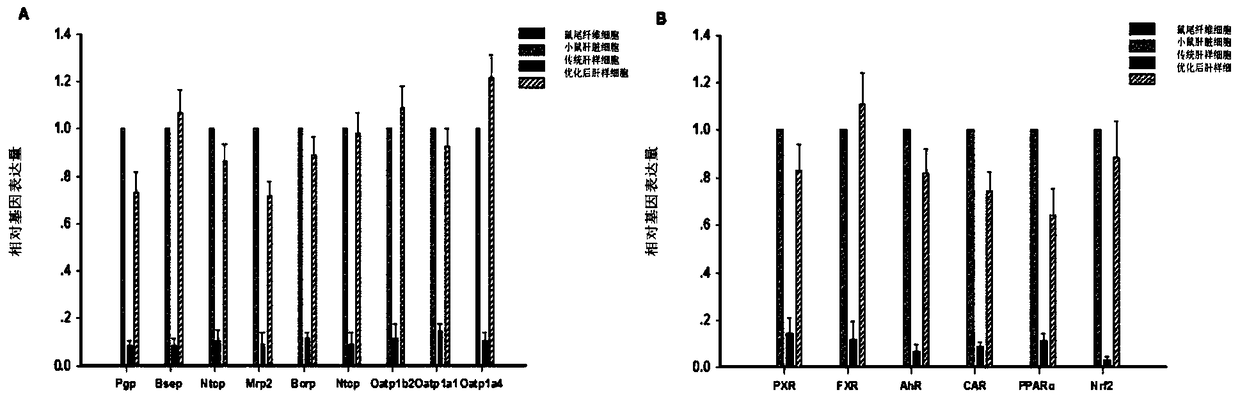
[0048] Example 1 Obtainment of liver-like cells after optimization of mouse source
[0049] Step 1. Using direct transdifferentiation technology to generate traditional mouse-derived liver-like cells
[0050] 1) Molecular cloning and lentivirus production
[0051] First construct the vector of the target gene: the multiple cloning site is inserted into the PmeI restriction enzyme site of the lentiviral vector pWPI with the reporter gene GFP, and the cDNAs of the three transcription factors of the target genes Gata, Hnf1α, and Foxa3 are respectively connected to this On the modified vector, three constructed pWPI vectors containing transcription factors and two packaging plasmids psPAX2 and pMD2.G were added to 293T cells. After 48 hours, the supernatant containing the lentivirus was collected and filtered through a 0.45 μm filter, aliquoted into EP tubes, and frozen at -80°C.
[0052] 2) Culture of mouse tail fibroblast (TFF) cells or mouse embryonic fibroblast (MEF) cells: ...
Embodiment 2
[0063] Example 2 Obtainment of hepatic-like cells after optimization of human source
[0064]In addition to replacing mouse tail fibroblast (TFF) cells or mouse embryonic fibroblast (MEF) cells with human embryonic fibroblasts (derived from maternal placenta samples), human embryonic fibroblasts (derived from Pregnant women's placenta samples), using a method similar to Example 1 to obtain optimized human-derived hepatic cells.
Embodiment 3
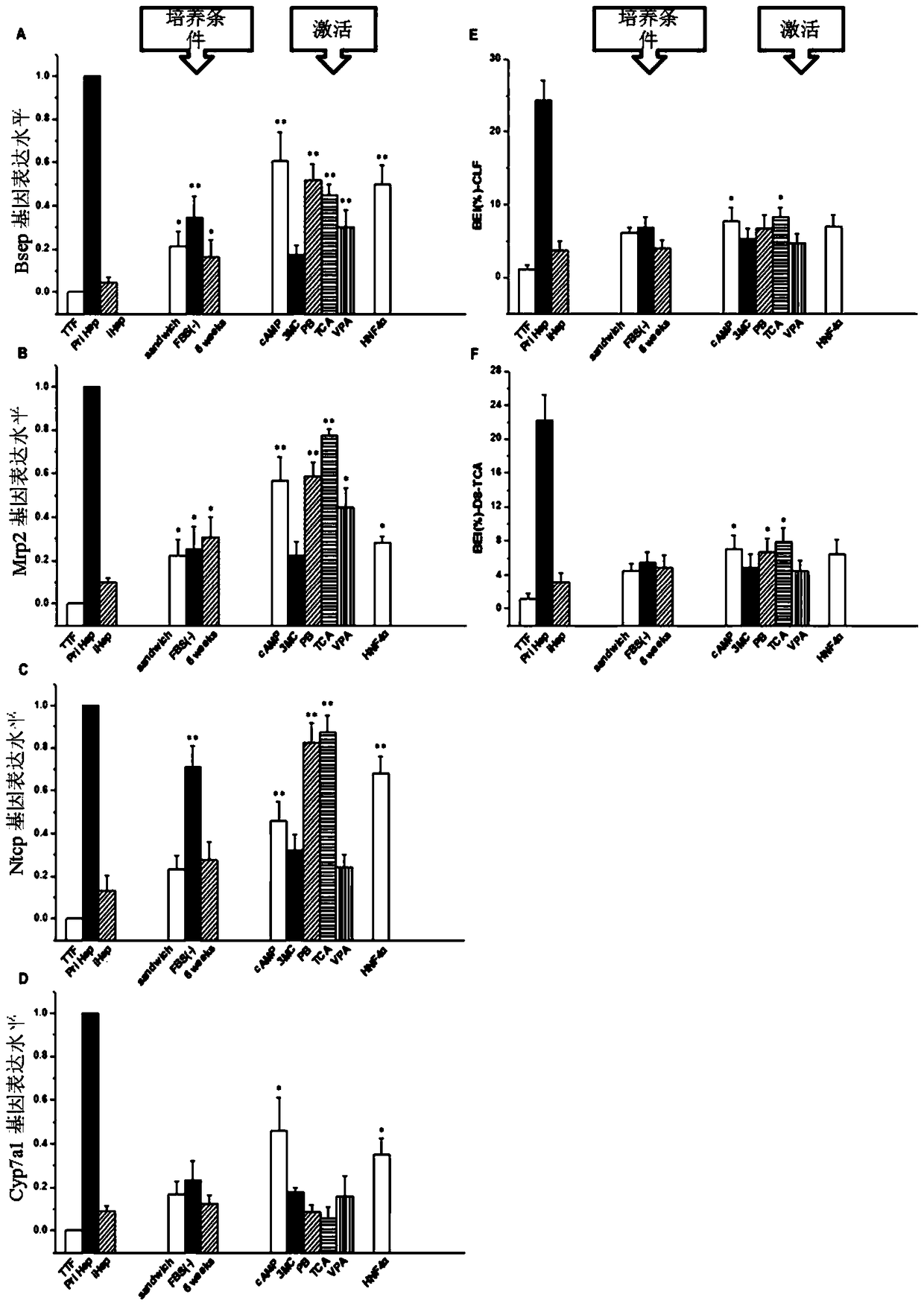
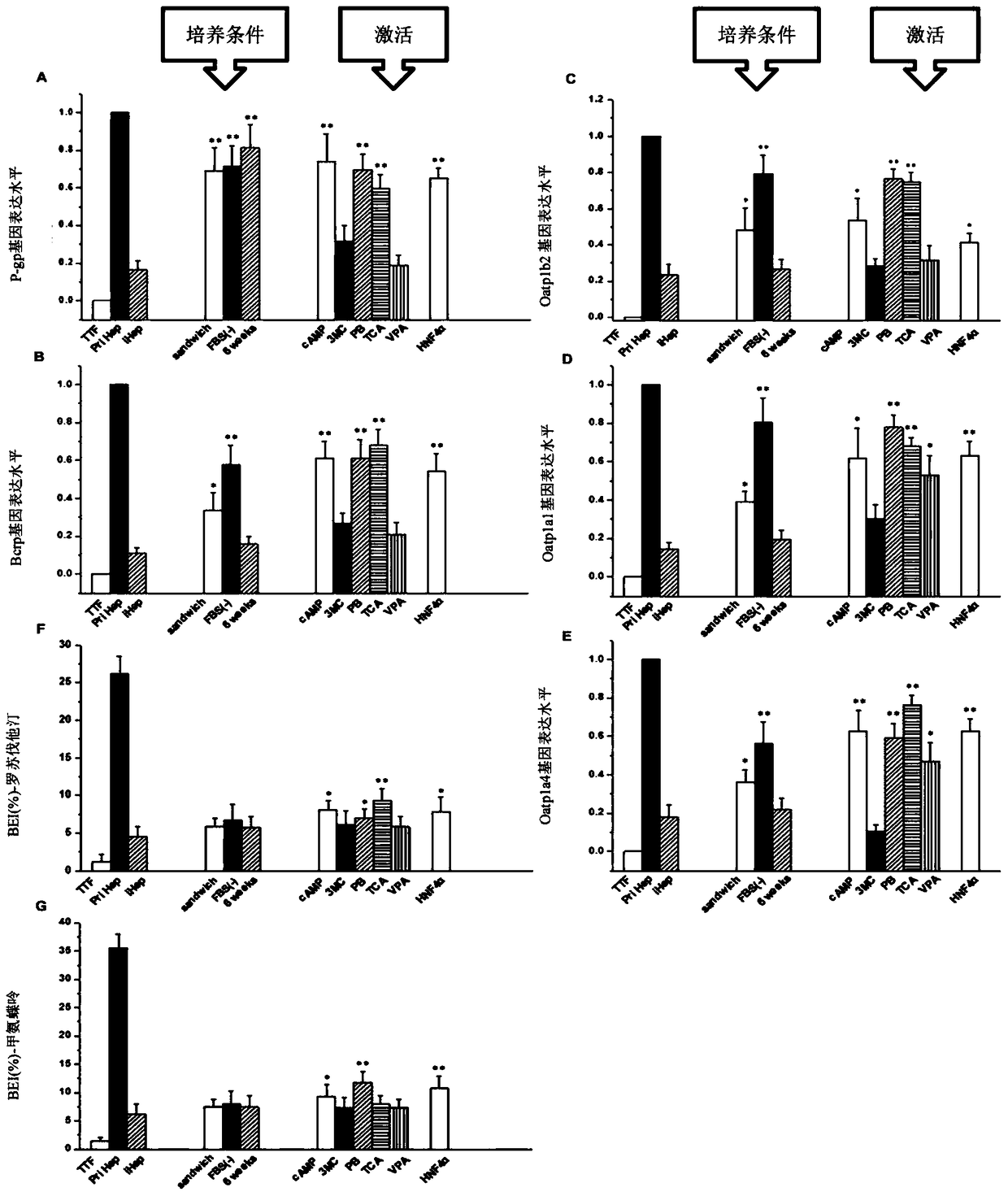
[0065] Example 3 An experimental study was conducted on combinations of nuclear receptor agonists of different concentrations and types.
[0066] Firstly, hepatic-like cells transfected with Hnf4α were obtained on the basis of traditional hepatic-like cells (the steps are the same as steps 1-3 in Example 1). Then switch to the induction medium containing different concentrations and different combinations of nuclear receptor agonists for optimal selection, try a single nuclear receptor and its concentration, multiple combinations of nuclear receptor agonists and their concentrations, and finally obtain Most preferred combinations and concentrations of nuclear receptor agonists. The specific results are shown in Tables 1, 2, and 3 below.
[0067] Table 1: Single nuclear receptor agonists and their concentration ranges
[0068]
[0069] Table 2: Various combinations of nuclear receptor agonists and their application concentrations
[0070]
[0071]
[0072] Table 3 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com