Patents
Literature
37 results about "Drug transporter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Caco-2 cell model for CRISPR/CAS9-mediated drug transporter targeted knockout and method thereof
The invention discloses a caco-2 cell model for CRISPR / CAS9-mediated drug transporter targeted knockout and a method thereof. The method is used for non-diagnostic or therapeutic purposes and comprises the following steps: designing sgRNA with target specificity for P-gp, BCRP and MRP2 transporters and constructing a sgRNA expression vector, wherein a sequence of the designed sgRNA is shown as SEQID NO. 1-6 in a sequence table; respectively designing P-gp, BCRP and MRP gene single knockout and pairwise combination double knockout by utilizing CRISPR / CAS9, co-transfecting a caco-2 cell with anhCas9 plasmid and performing monoclonal expansion culture to obtain the caco-2 cell model for transporter gene targeting. The caco-2 cell model obtained by the method disclosed by the invention has the beneficial effects that the mutual interference among different transporters is effectively eliminated, and a more specific and more sensitive cell model is provided for drug transport research.
Owner:SOUTH CHINA UNIV OF TECH
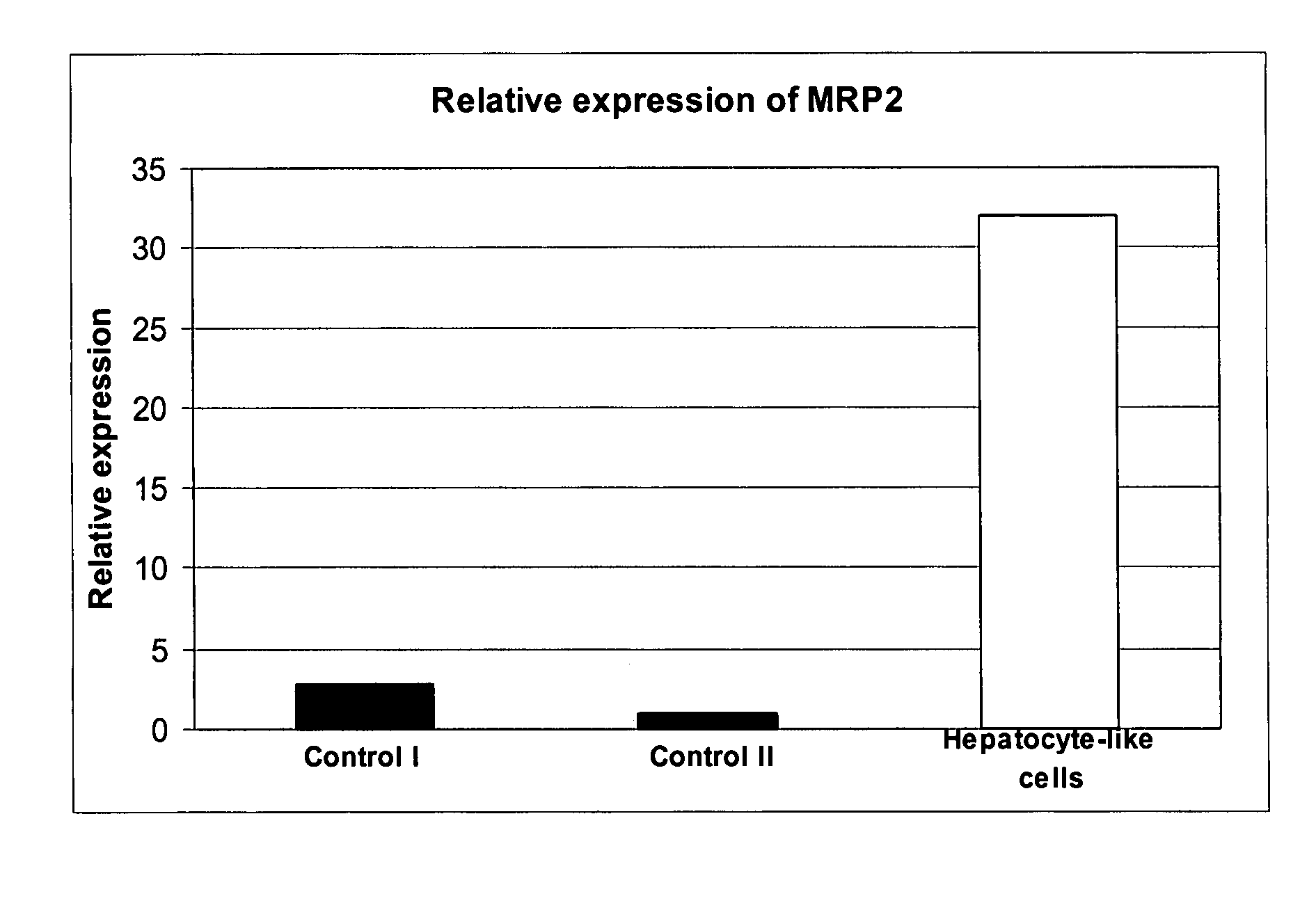
Novel hepatocyte-like cells and hepatoblast-like cells derived from hBS cells
InactiveUS20080019950A1Improve predictabilityReduce needBiocideHepatocytesIn vitro studyMother cells
The present invention relates to a novel hepatocyte-like cell population derived from hBS cells and to the potential use of such heopatocyte-like cells in e.g. medical treatment, drug screening and toxicity testing. Furthermore, the invention relates to hepatoblast-like cells that may have suitable characteristics so that they can be used for the same applications as the hepatocyte-like cells and that furthermore may be used in in vitro studies of hepatogenesis such as early hepatogenesis or hepato-regenerative disorders. Both the hepatocyte-like and the hepatoblast-like cells according to the invention express drug transporter and / or drug metabolising characteristics either at the gene or protein expression level.
Owner:CELLARTIS AB (SE)
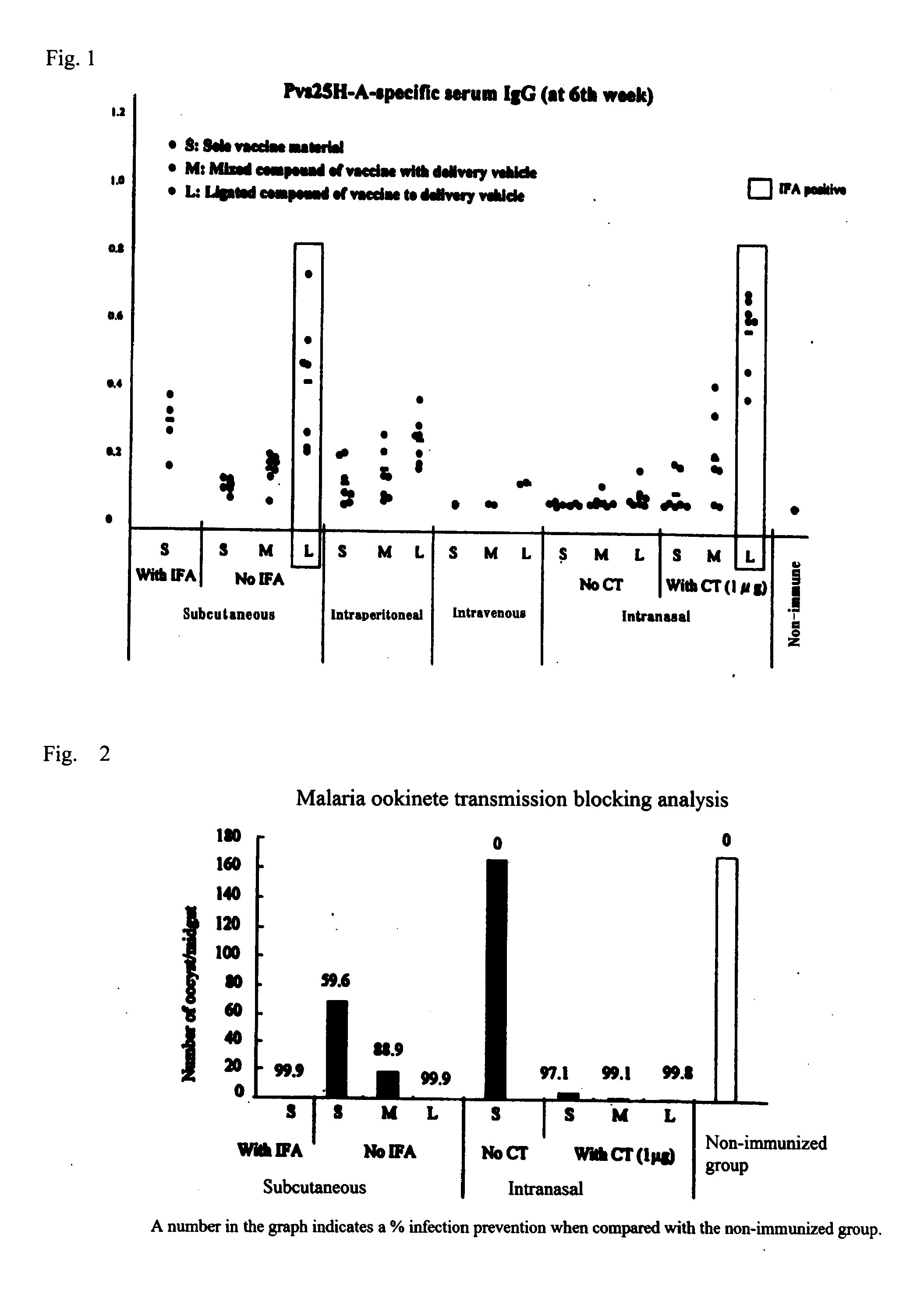
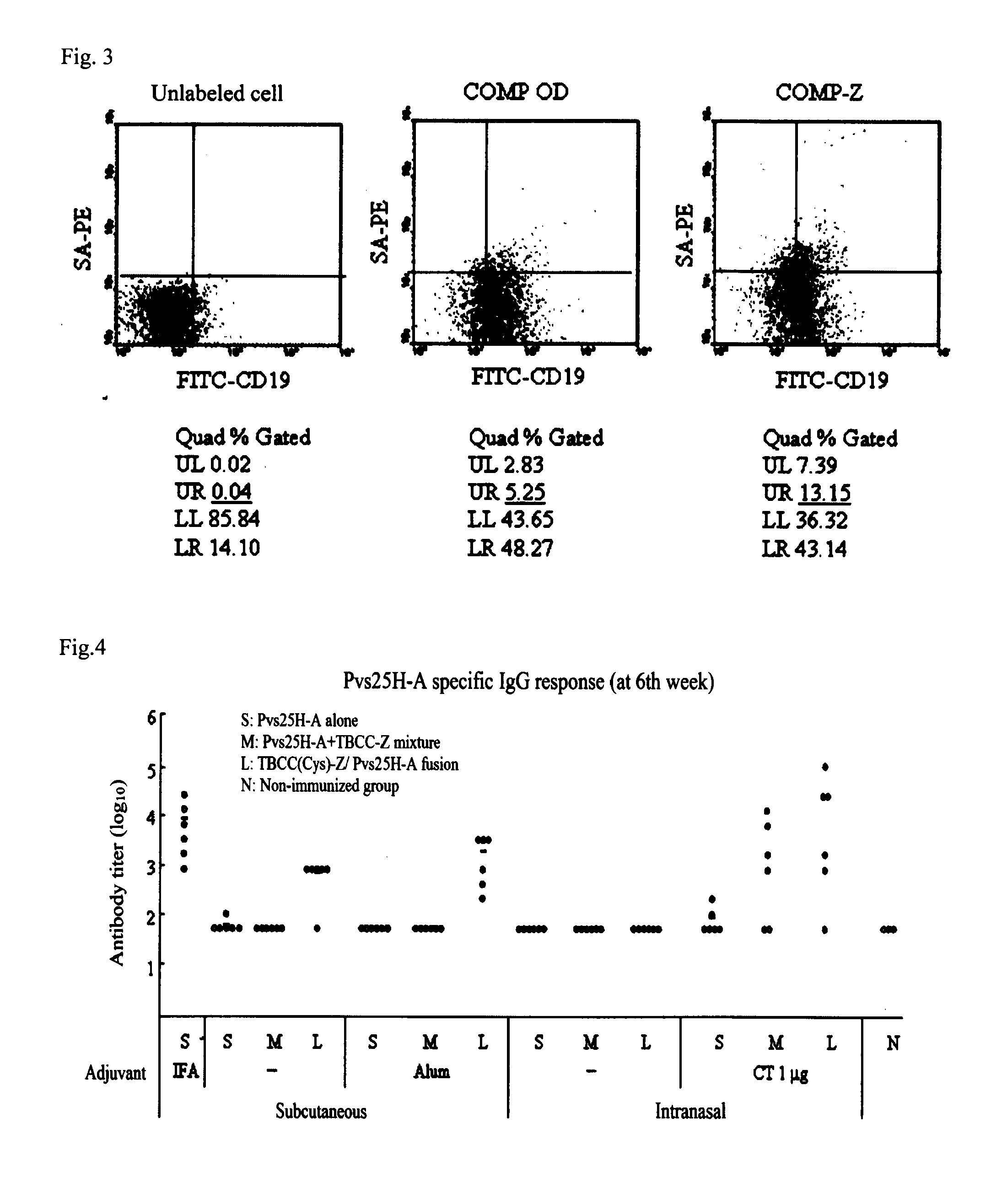
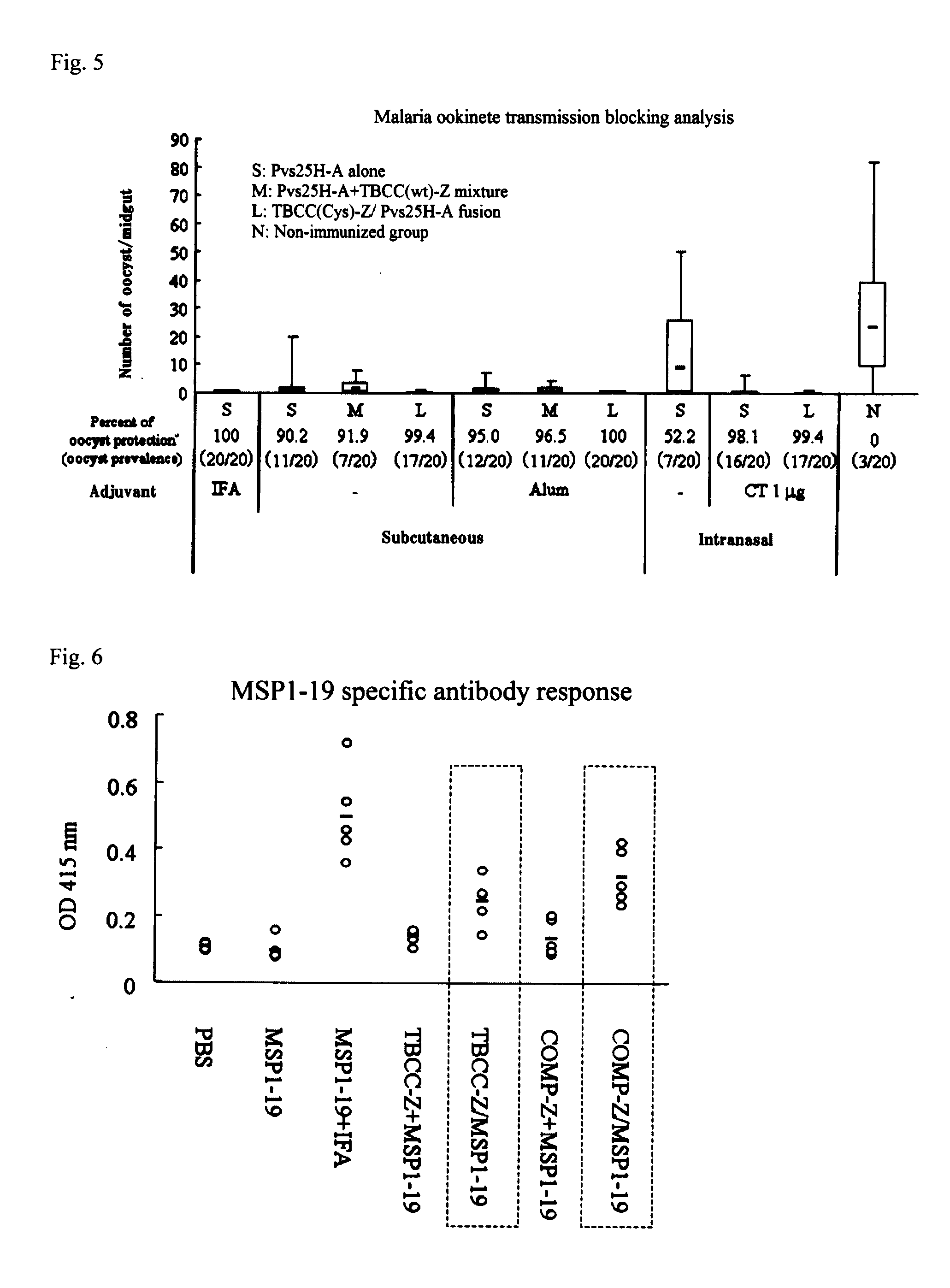
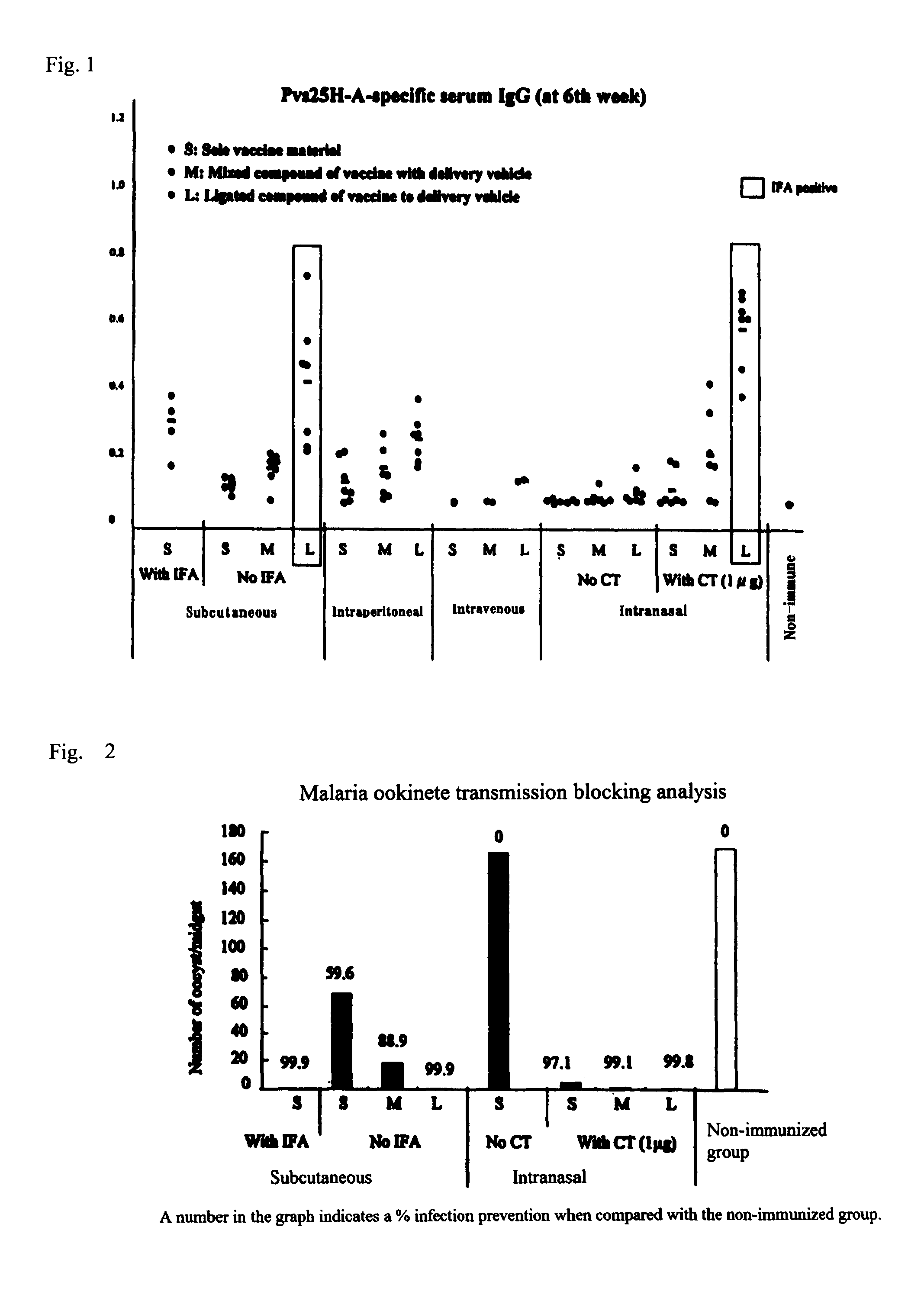
Drug transporter, and adjuvant and vaccine each utilizing same
InactiveUS20120100165A1Good effectImproving immunogenicityAntibody mimetics/scaffoldsAntiviralsAdjuvantImmunogenicity
An objective of the invention is to provide a drug delivery vehicle capable of allowing a vaccine or adjuvant to reach a target cell or tissue efficiently while being capable of improving the immunogenicity of the vaccine or capable of enhancing the immunostimulating effect of the adjuvant as well as a vaccine or adjuvant utilizing the same. Said drug delivery vehicle contains a multimeric protein having a coiled coil structure and a ligand molecule to a receptor of an immune cell.
Owner:UNIVERSITY OF THE RYUKYUS
In-vitro hepatocyte-like cell culture method and optimized hepatocyte-like cell cultured by the method
ActiveCN104694456AThe correlation of secretion rate is goodHas a secretory functionDigestive systemArtificial cell constructsSerum igeSerum free
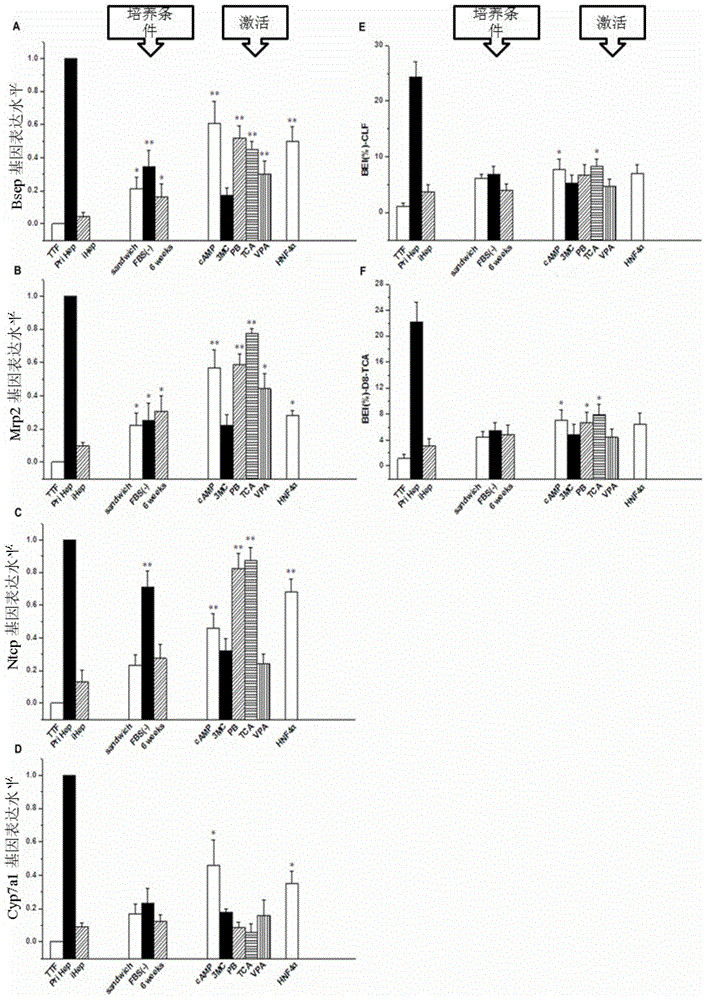
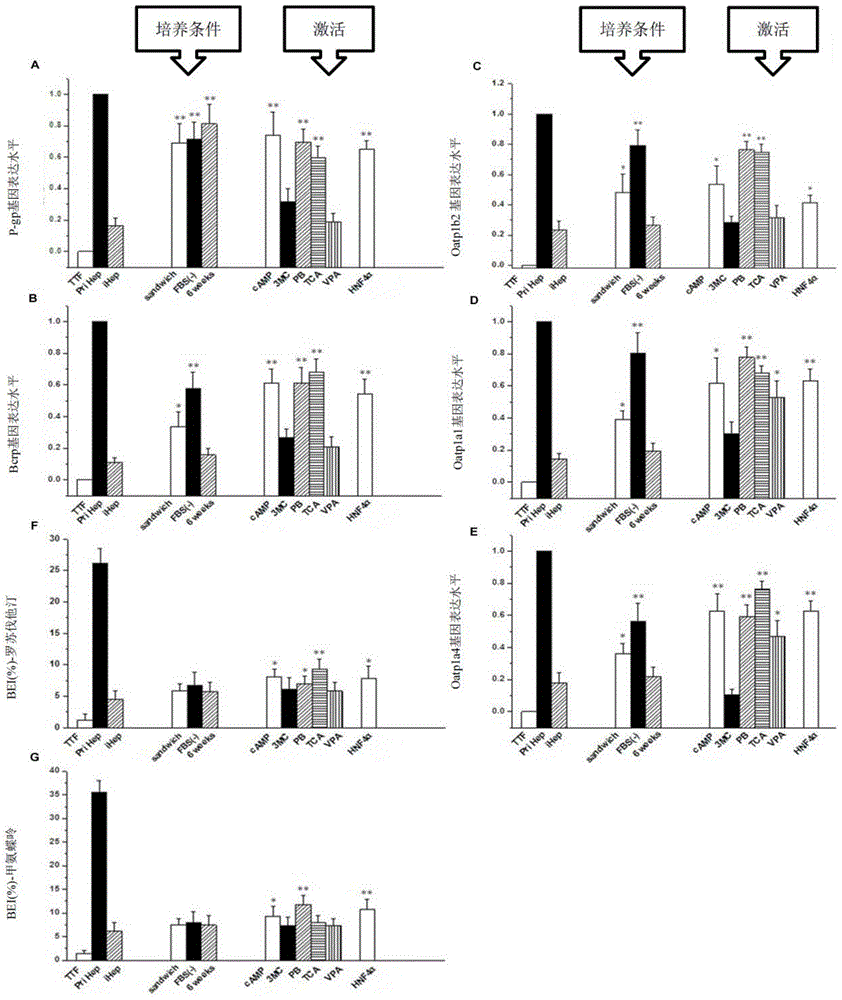
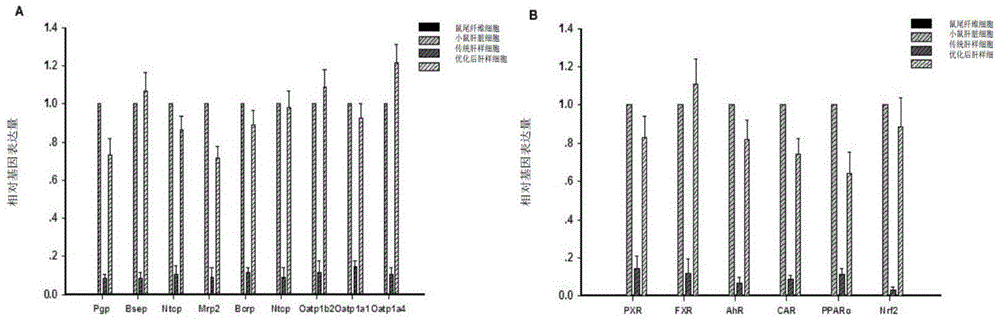
Provided are an in vitro culturing method for obtaining optimized hepatocyte-like cells and optimized hepatocyte-like cells obtained by using this method. The in vitro culturing method comprises the use of a sandwich cell culture method, serum-free culturing and an induction medium. The optimized hepatocyte-like cells obtained above have a structure and function close to that of primary liver cells, and the expression levels of the drug transporter, bile acid transporter and / or bile acid synthase thereof are significantly higher than that of traditional hepatocyte-like cells; the drug bile secretion index (BEI) and intrinsic biliary clearance (CL b,int) thereof are significantly higher than that of traditional hepatocyte-like cells; and the polarized expression of the drug transporter appears therein.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Inhibitors of ABC drug transporters at the blood-brain barrier
InactiveUS7034036B2Reduce outflowImprove concentrationBiocideAnimal repellantsActive agentBrain concentrations
The present invention relates to inhibitors of drug transporters of the ABC protein superfamily, particularly transporters present at the blood brain barrier. ABC transporter inhibitors identified according to the invention increase brain concentrations of CNS-active agents. Such inhibitors increase the influx into the brain and / or reduce the efflux from the brain of such CNS-active agents.
Owner:PAIN THERAPEUTICS INC
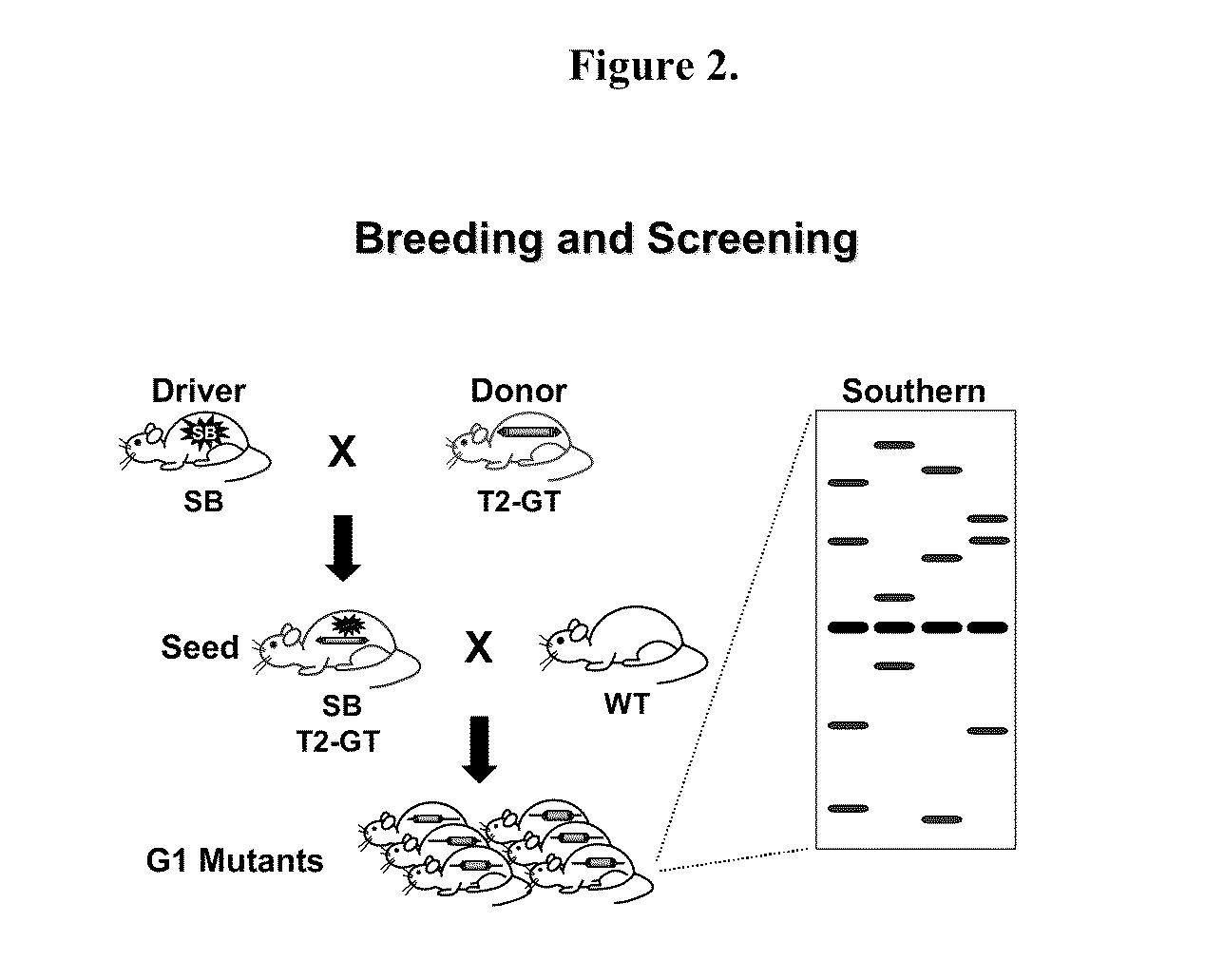
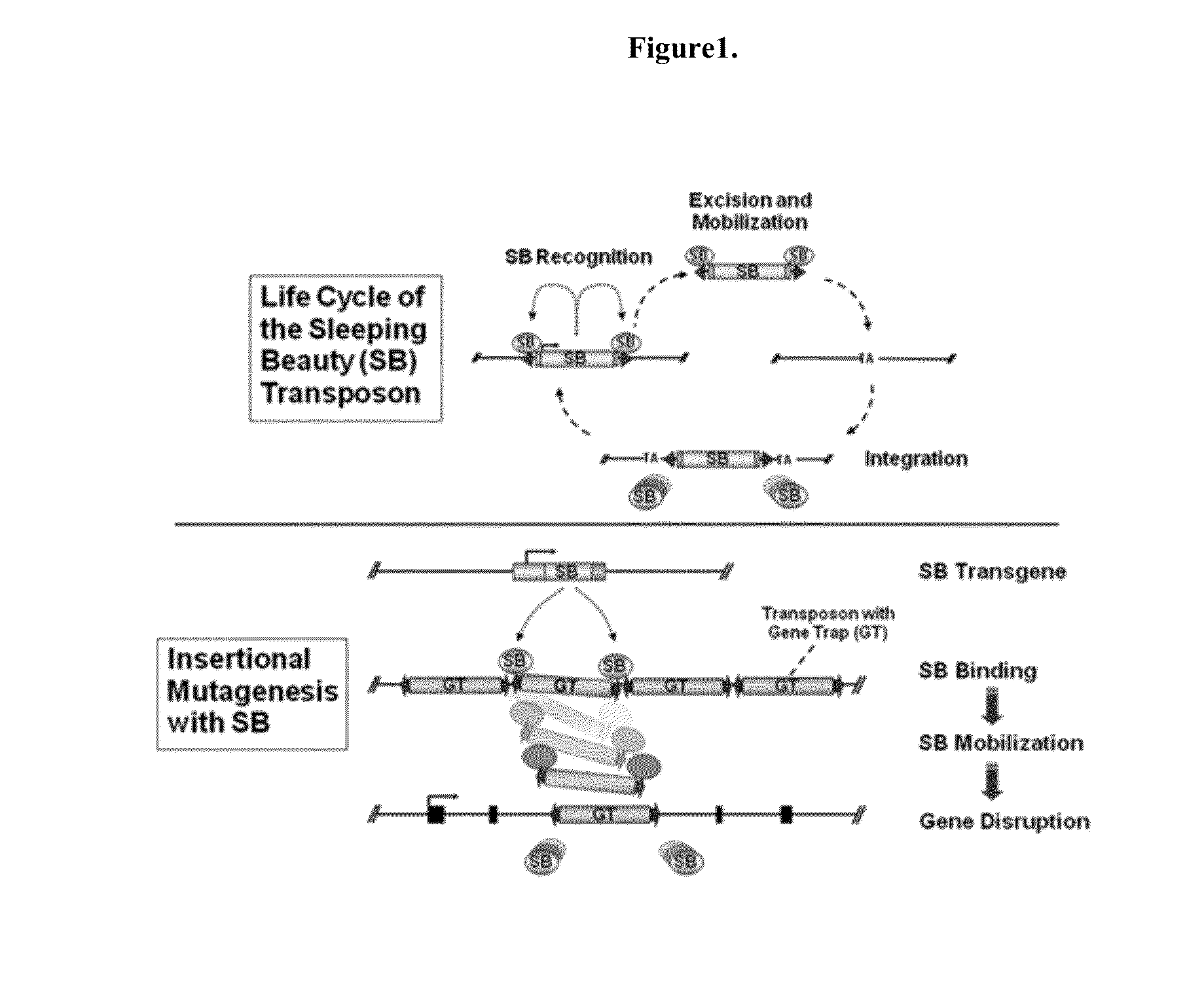
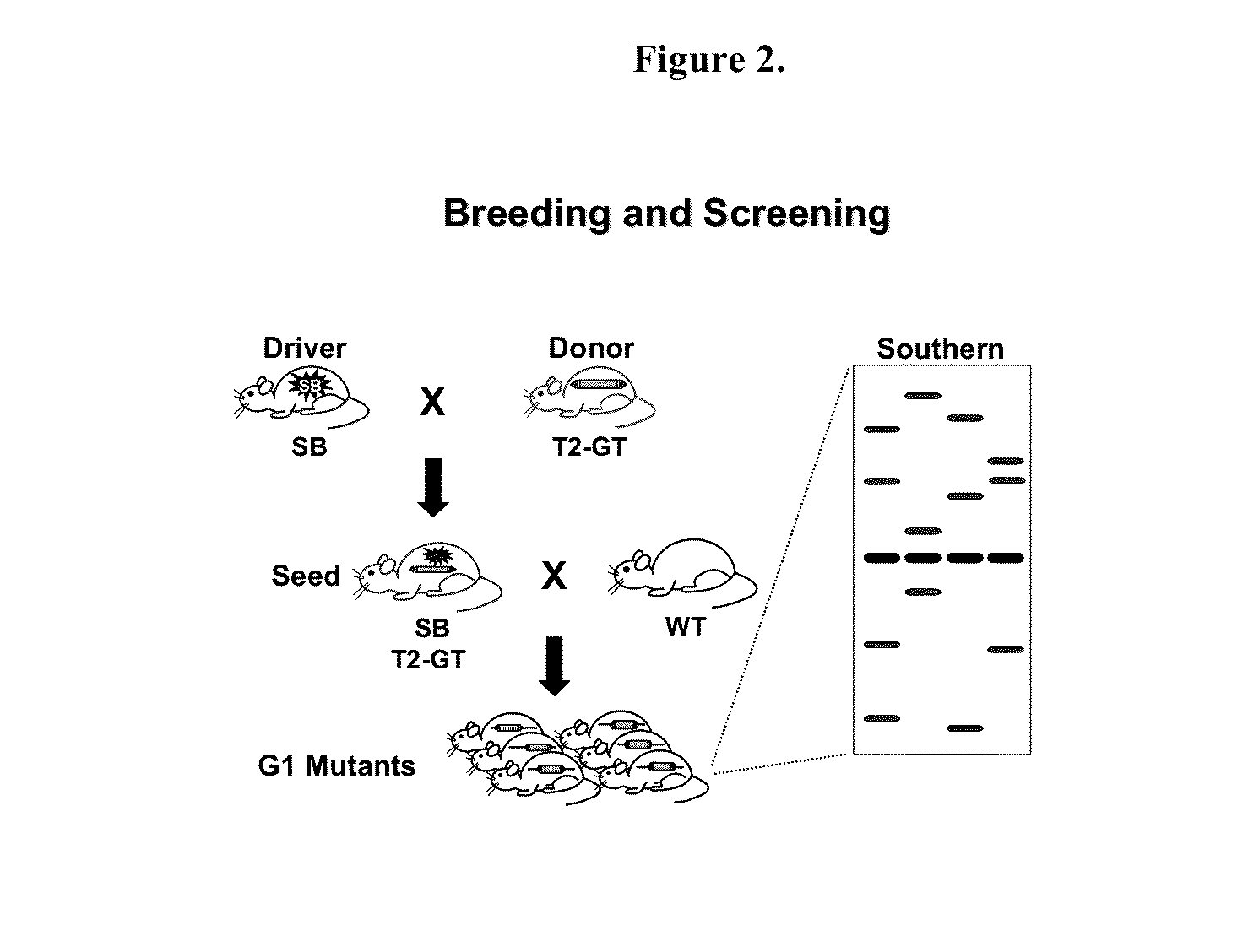
Genetically Modified Rat Models for Pharmacokinetics
The present invention provides a desired rat or a rat cell which contains a predefined, specific and desired alteration rendering the rat or rat cell predisposed to drug transport sensitivity or resistance drug transport resistance or sensitivity. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a drug transporter gene such as the Slc7a11 (NC_005101.2) gene, the Abcb1 (NC_005103.2) gene, etc. The present invention also provides a desired rat or a rat cell which contains a predefined, specific and desired alteration rendering the rat or rat cell predisposed to drug transport sensitivity or resistance drug transport resistance or sensitivity. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a drug transporter gene.
Owner:TRANSPOSAGEN BIOPHARM
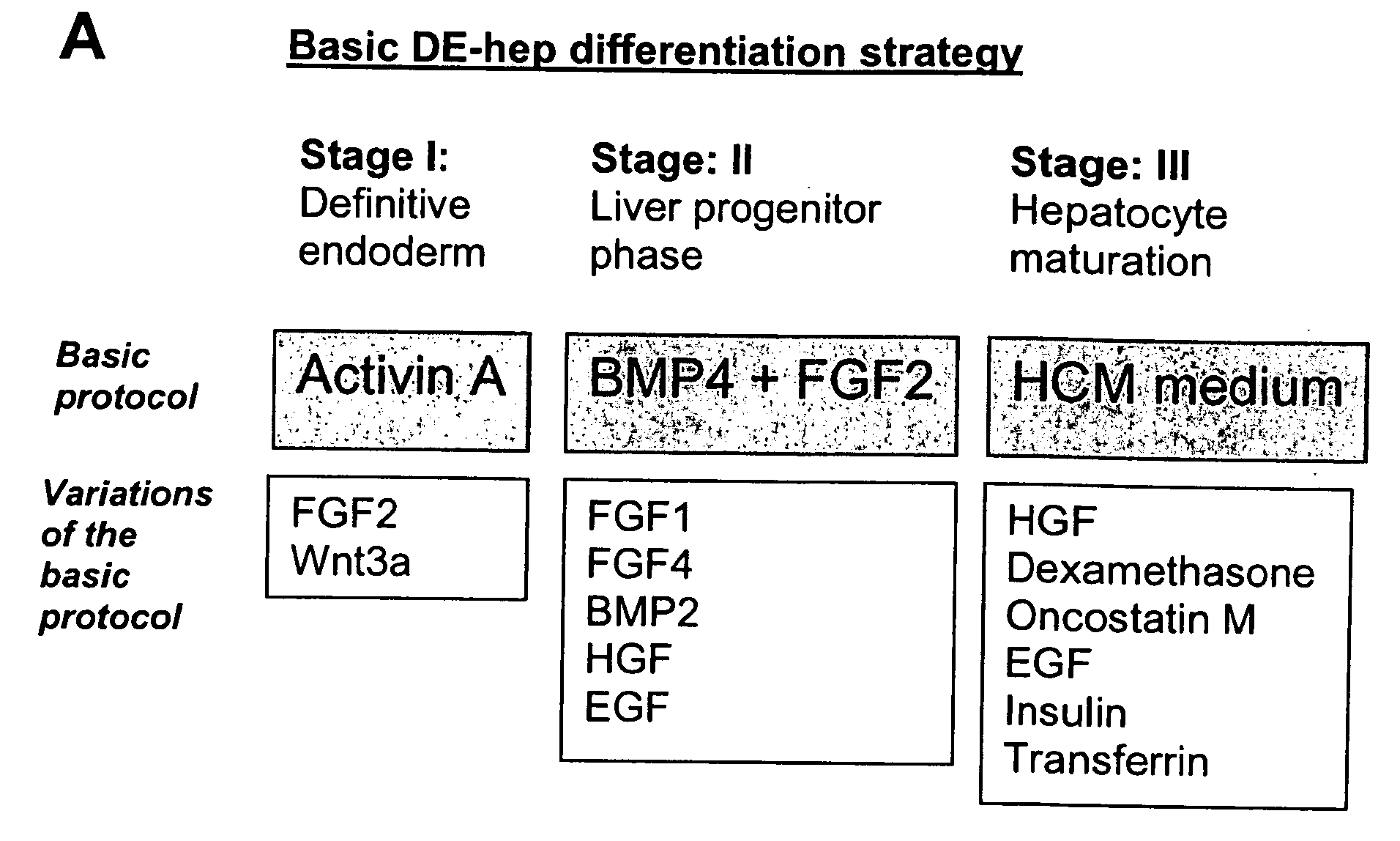
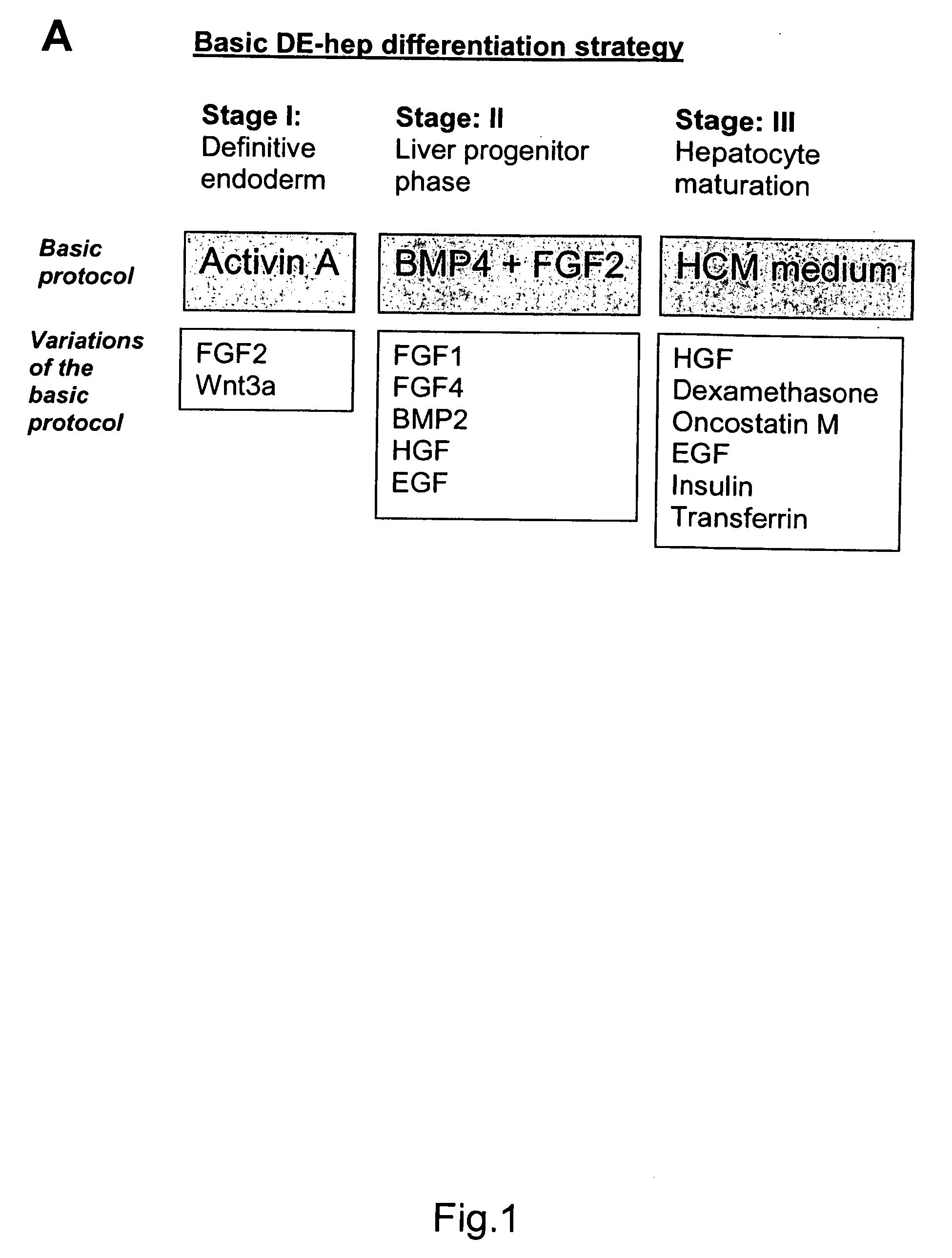
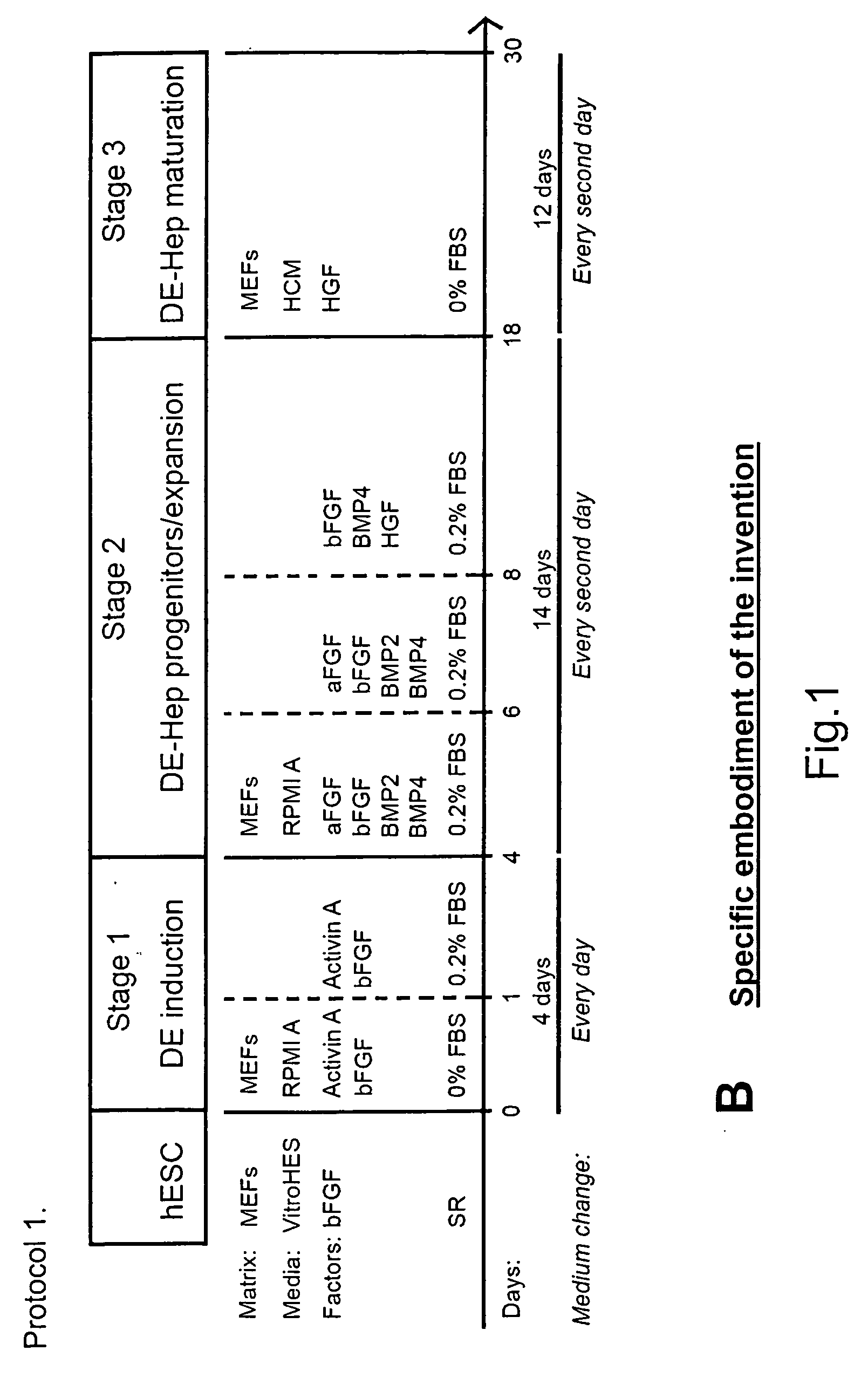
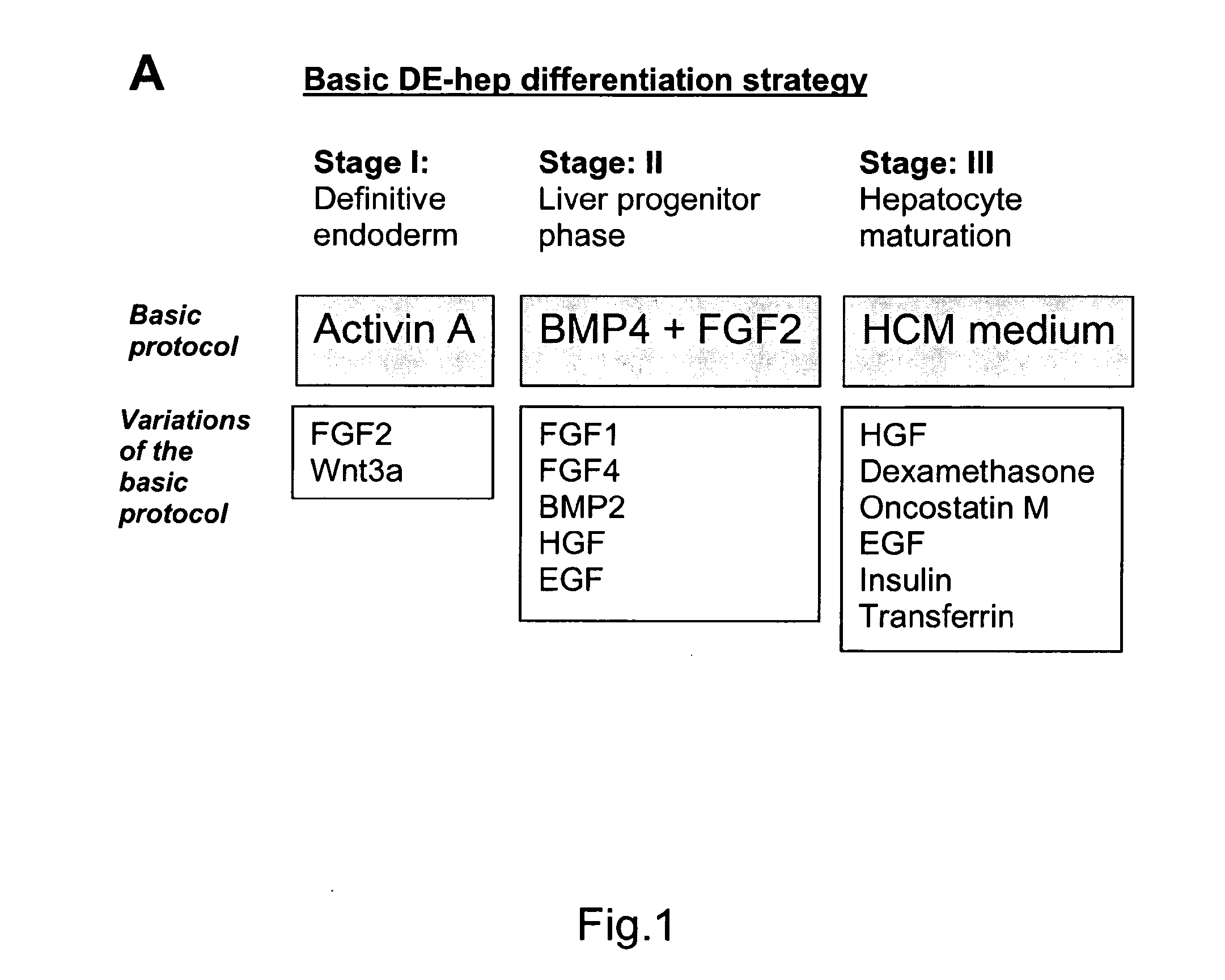
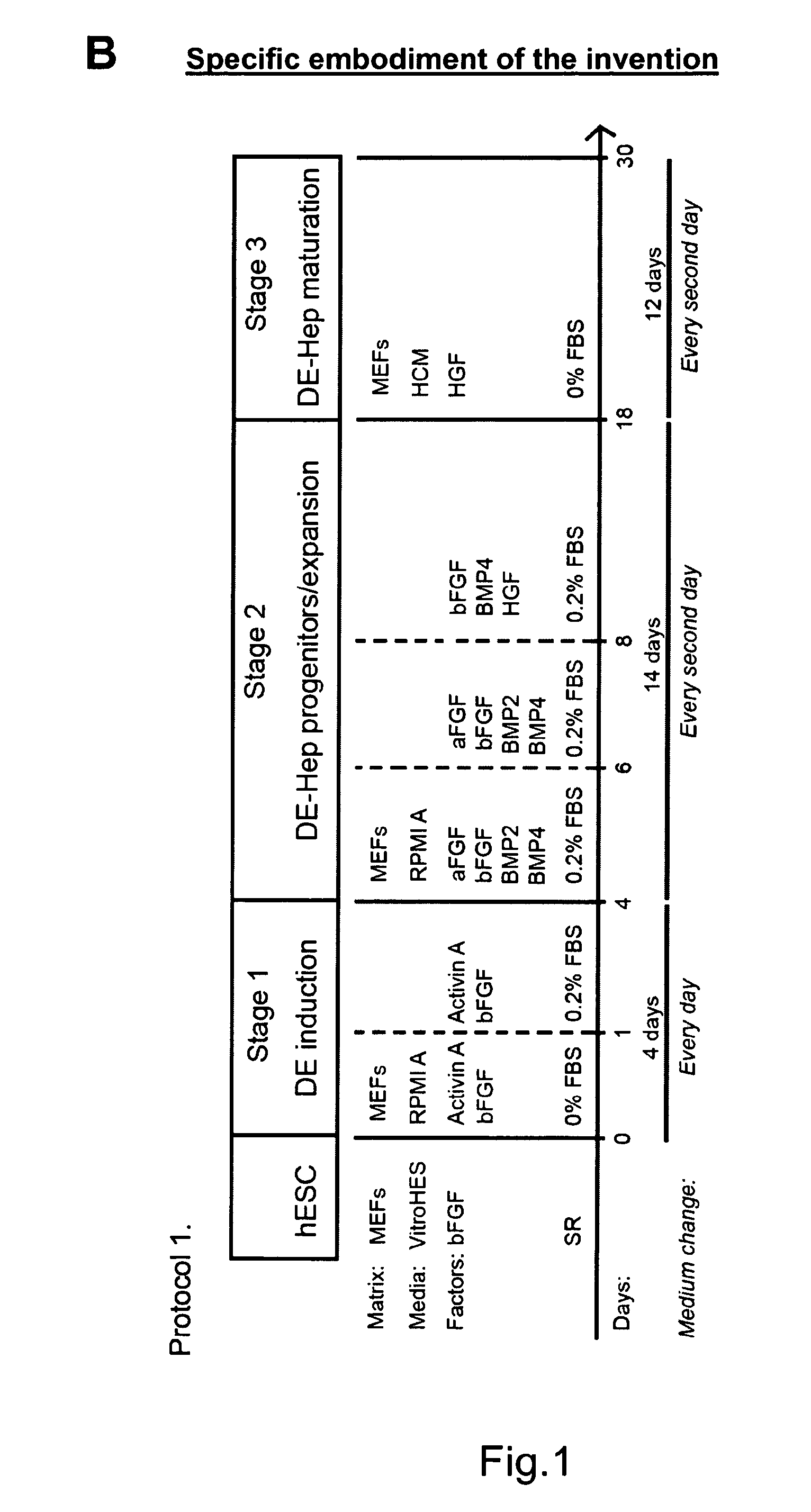
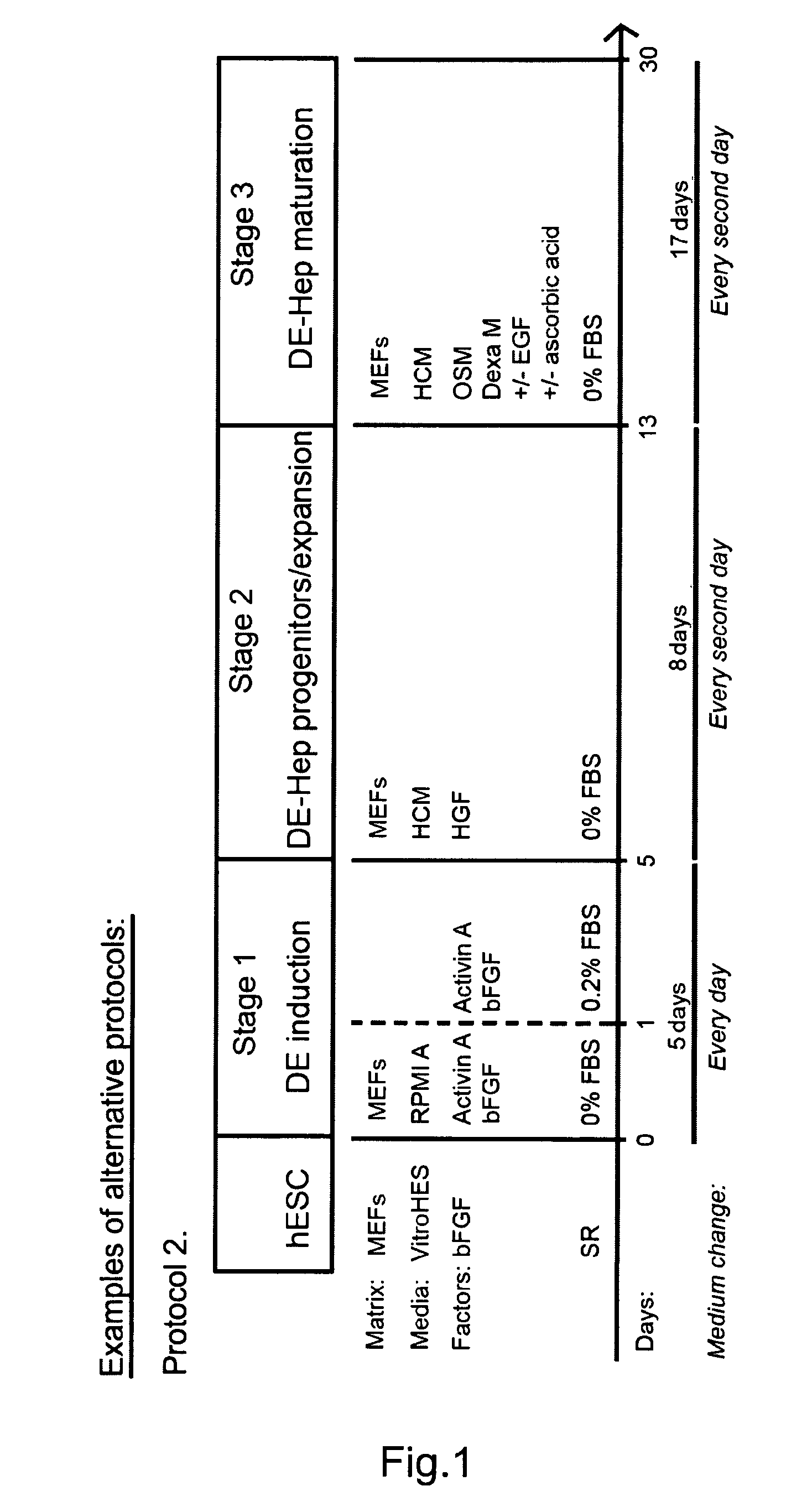
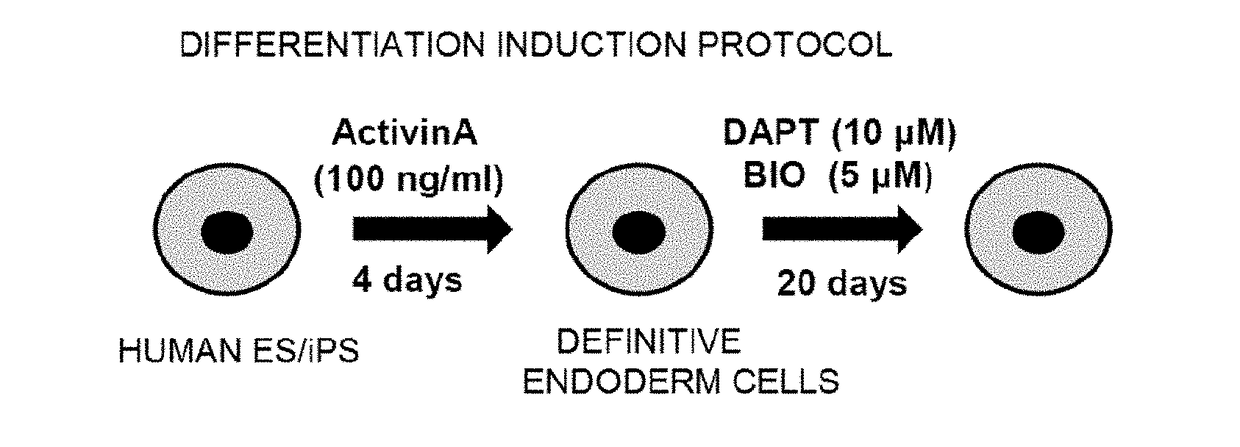
Novel population of hepatocytes derived via definitive endoderm (DE-hep) from human blastocysts derived stem cells
InactiveUS20090123432A1Increase percentageEasy to convertBiocideMicrobiological testing/measurementGerm layerMetabolizing enzymes
The present invention relates to a novel hepatocyte-like cell progenitor and / or a novel hepatocyte-like cell derived via definitive endoderm from human blastocyst-derived stem (hBS) cells, to a method for the preparation of such cells and to the potential use of such cells in e.g. pharmaceutical drug discovery and development, toxicity testing, cell therapy and medical treatment.In particular is presented a definitive endoderm derived hepatocyte-like cell with important liver-expressed marker genes and important metabolizing enzymes, as well as drug transporters.
Owner:TAKARA BIO EURO
Genetically modified rat models for pharmacokinetics
InactiveUS20150052623A1Compound screeningCell receptors/surface-antigens/surface-determinantsRat modelDrug transport
Owner:OSTERTAG ERIC M +1
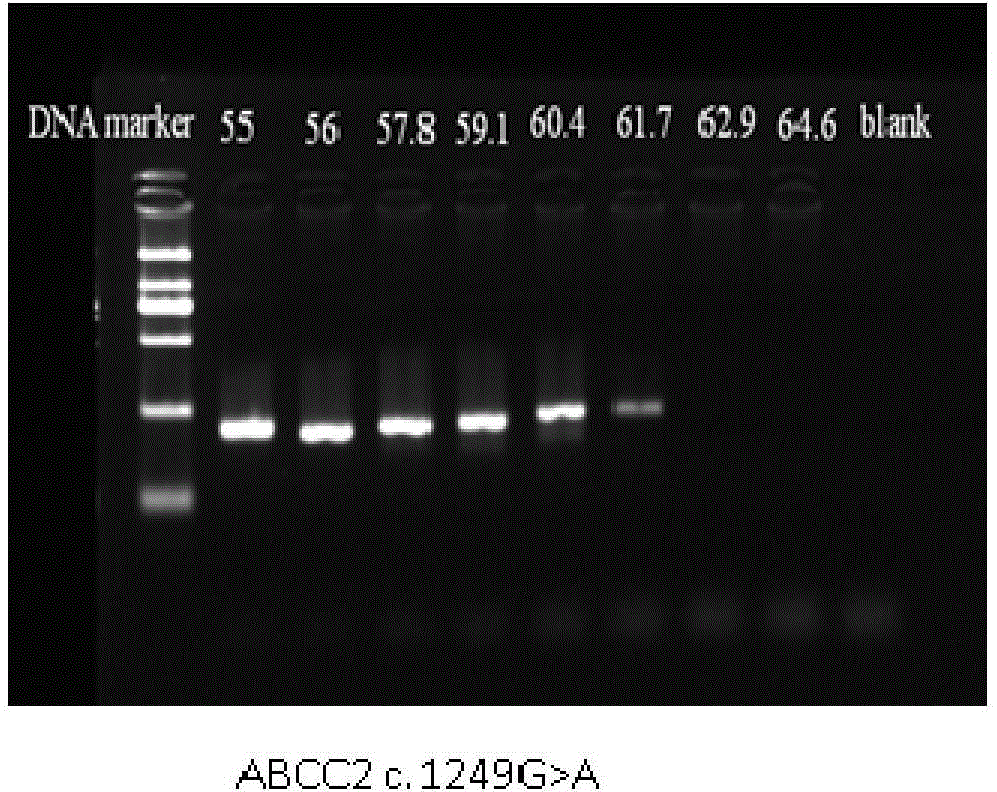
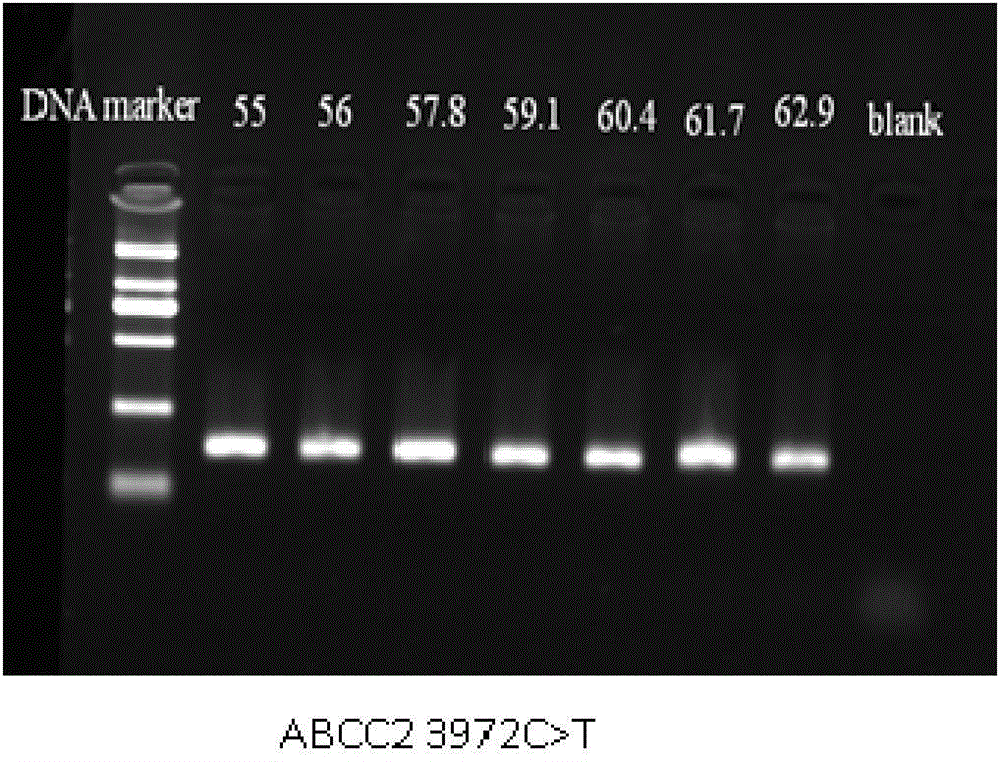
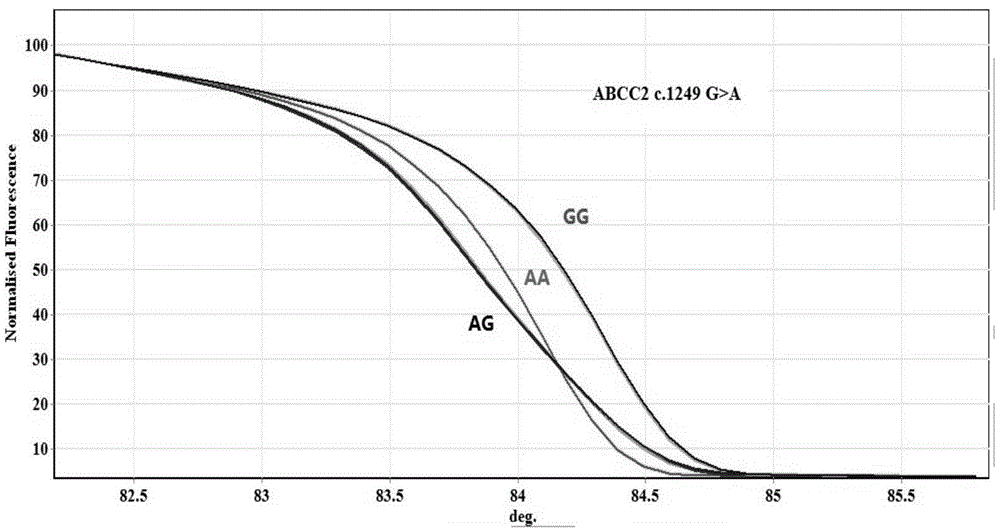
Method for measuring ABCC2 gene polymorphism
InactiveCN105316401AAvoid human contactReduce pollutionMicrobiological testing/measurementFluorescenceContamination
The invention belongs to the field of molecular biological techniques and medical examination, and relates to a device for detecting drug transporter ABCC2 gene polymorphism, in particular to a method for detecting ABCC2 c. 1249 G>A and ABCC 2 3972>T gene polymorphism. After DNA of a sample is extracted, conventional PCR (polymerase chain reaction) amplification (containing fluorescent dye) is carried out, and a melting curve with high resolution ratio is used for analyzing genetic typing of the sample. The method is easy and convenient to operate, a sequence specificity probe is not required, the method is not limited by basic sites, meanwhile, cross contamination is avoided by closed tube operation, and the method has the features of high efficiency, quickness, sensitivity, low cost and high flux, is suitable for clinical conventional gene detection, and further provides a technical support for clinical individual rational drug use.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
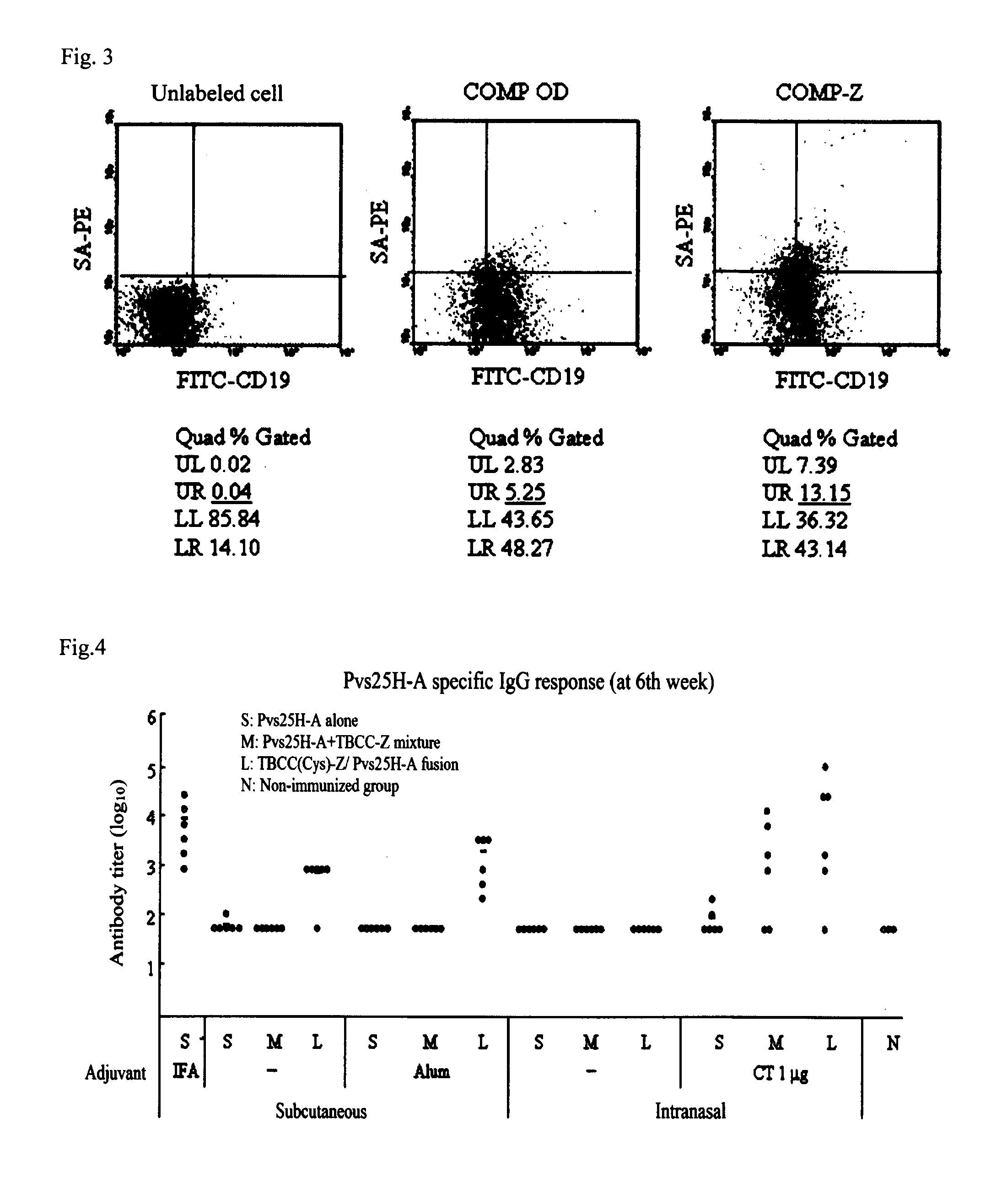
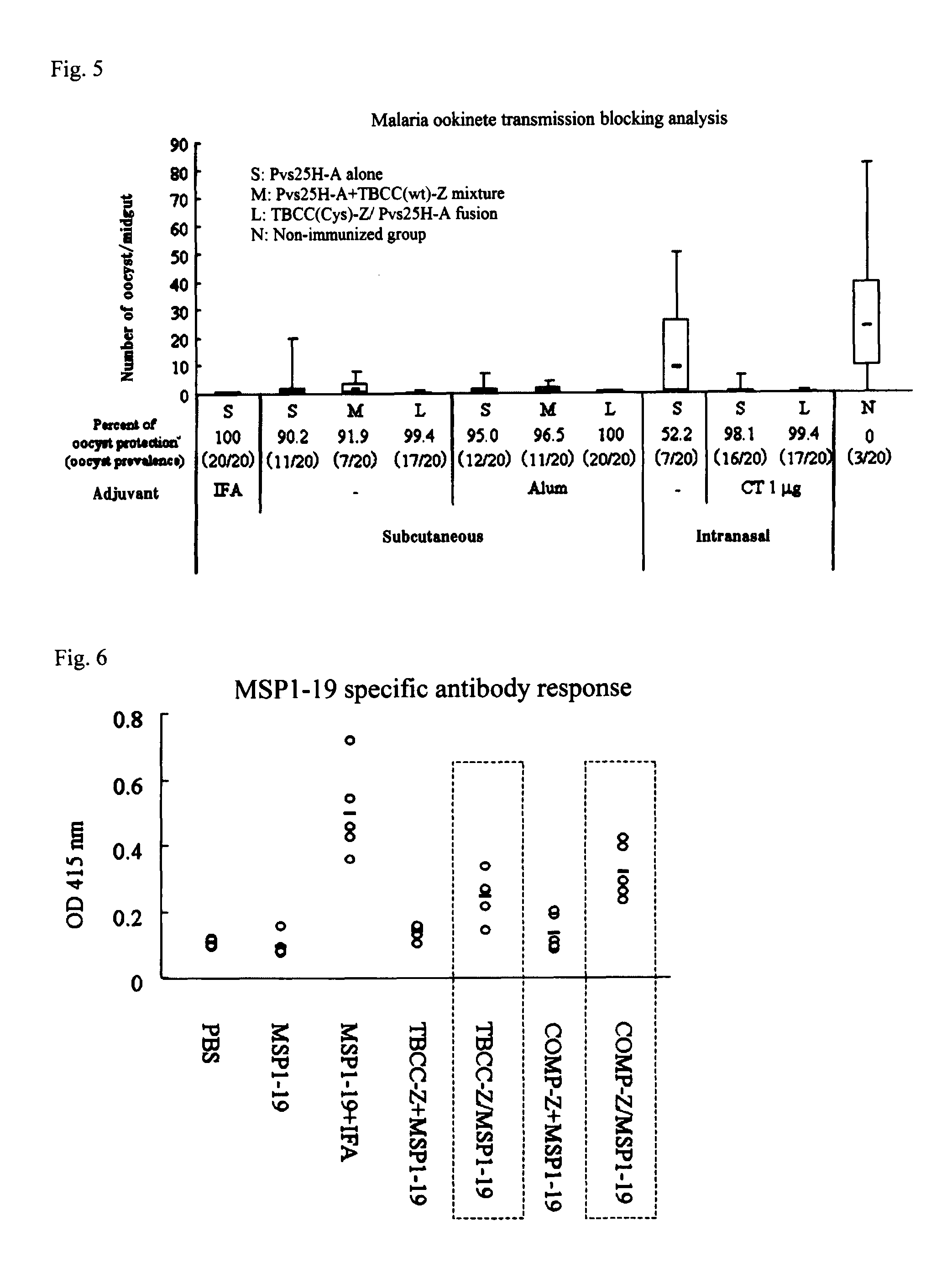
Drug transporter, and adjuvant and vaccine each utilizing same
InactiveUS8580274B2Simple designImproving immunogenicityAntibody mimetics/scaffoldsAntiviralsAdjuvantImmunogenicity
An objective of the invention is to provide a drug delivery vehicle capable of allowing a vaccine or adjuvant to reach a target cell or tissue efficiently while being capable of improving the immunogenicity of the vaccine or capable of enhancing the immunostimulating effect of the adjuvant as well as a vaccine or adjuvant utilizing the same. Said drug delivery vehicle contains a multimeric protein having a coiled coil structure and a ligand molecule to a receptor of an immune cell.
Owner:UNIVERSITY OF THE RYUKYUS
Novel population of hepatocytes derived via definitive endoderm (de-hep) from human blastocysts stem cells
InactiveUS20100190202A1Easy to convertRegulating trafficMicrobiological testing/measurementDigestive systemGerm layerMetabolizing enzymes
The present disclosure relates to a novel hepatocyte-like cell progenitor and / or a novel hepatocyte-like cell derived via definitive endoderm from human blastocyst-derived stem (hBS) cells, to a method for the preparation of such cells and to the potential use of such cells in, e.g., pharmaceutical drug discovery and development, toxicity testing, cell therapy and medical treatment. In particular is presented a definitive endoderm derived hepatocyte-like cell with important liver-expressed marker genes and important metabolizing enzymes, as well as drug transporters.
Owner:CELLARTIS AB (SE)
Novel hepatocyte-like cells and hepatoblast-like cells derived from hbs cells
The present invention relates to a novel hepatocyte-like cell population derived from hBS cells and to the potential use of such heopatocyte-like cells in e.g. medical treatment, drug screening and toxicity testing. Furthermore, the invention relates to hepatoblast-like cells that may have suitable characteristics so that they can be used for the same applications as the hepatocyte-like cells and that furthermore may be used in in vitro studies of hepatogenesis such as early hepatogenesis or hepato-regenerative disorders. Both the hepatocyte-like and the hepatoblast-like cells according to the invention express drug transporter and / or drug metabolising characteristics either at the gene or protein expression level.
Owner:CELLECTIS SA
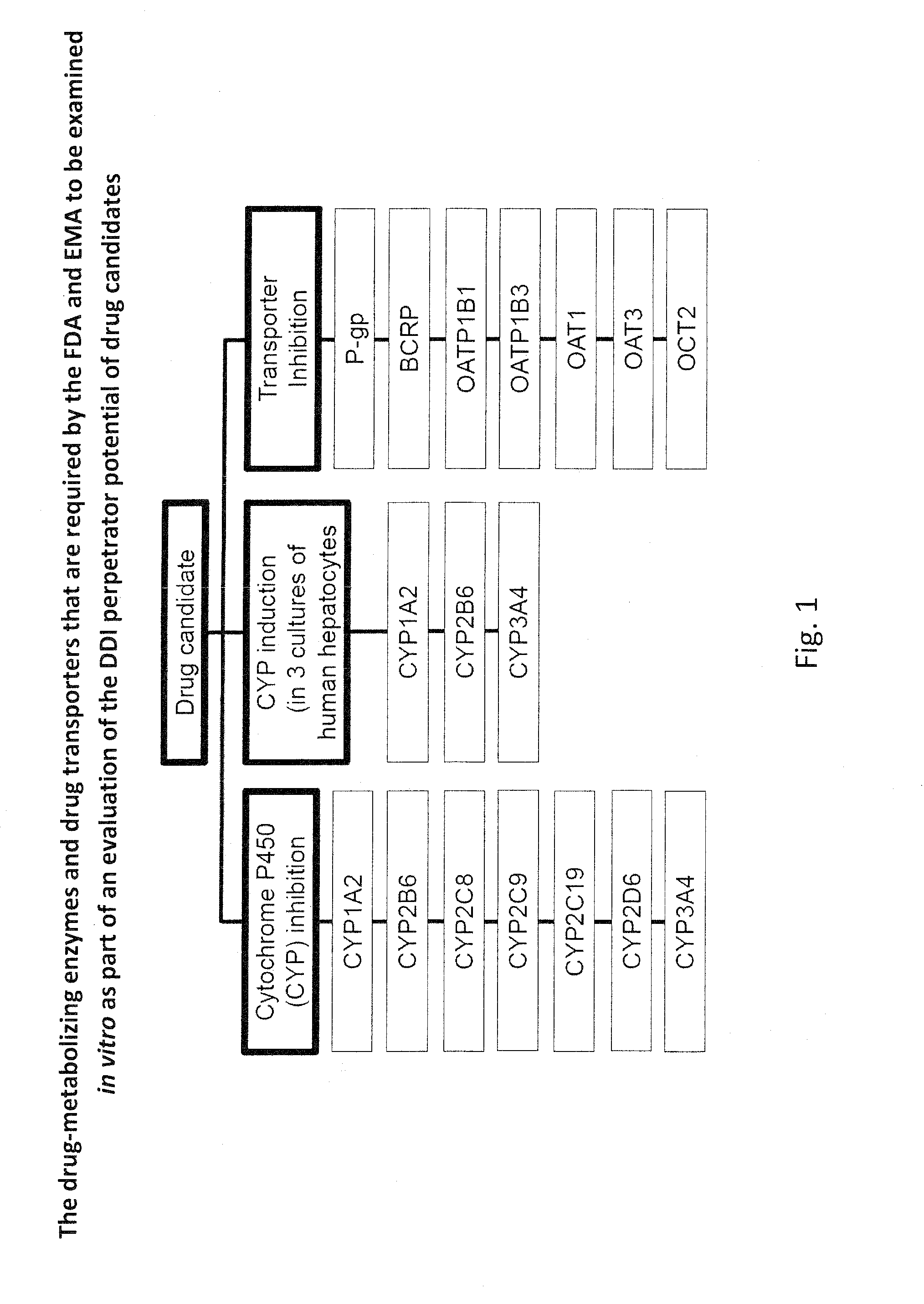
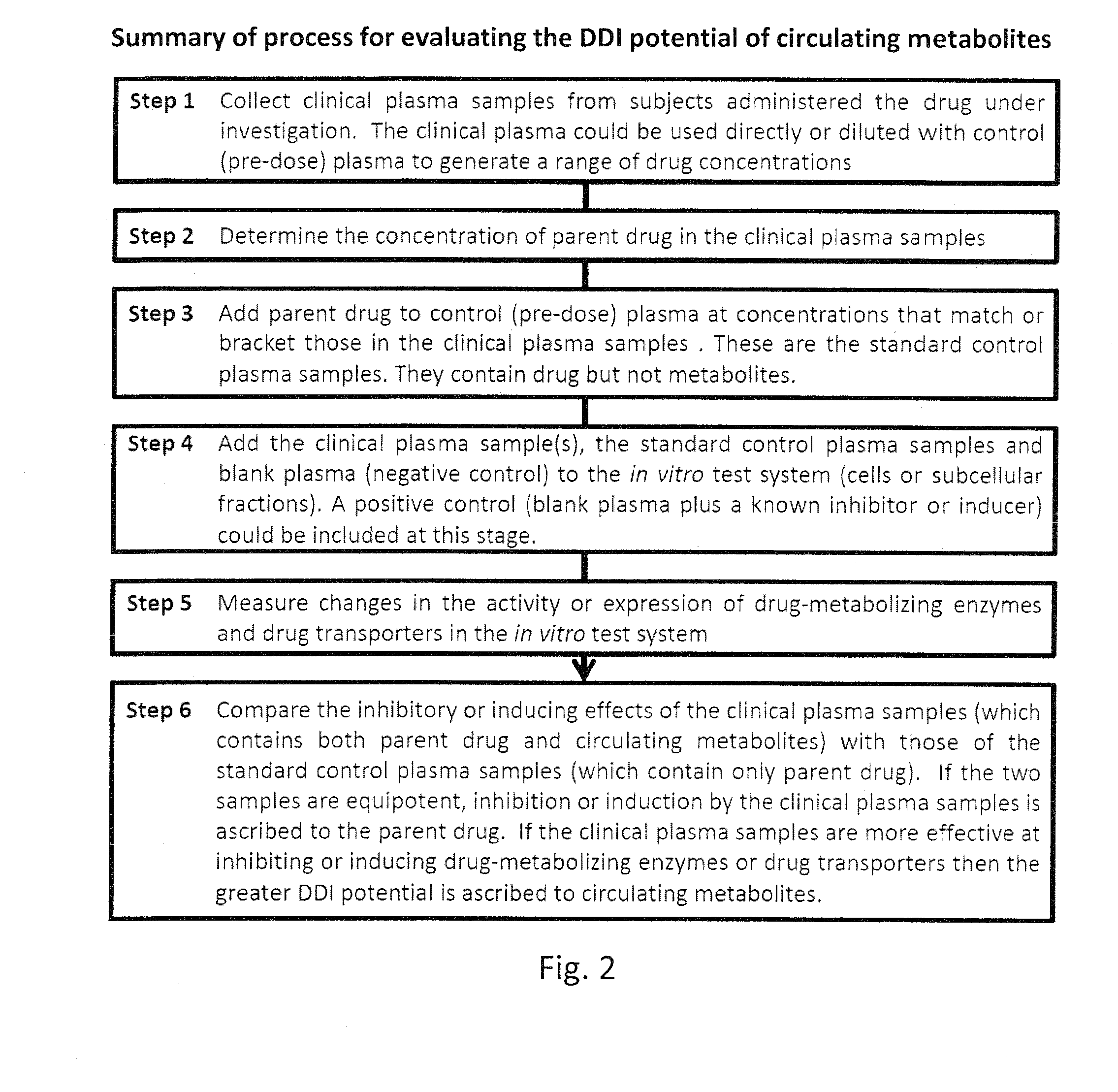
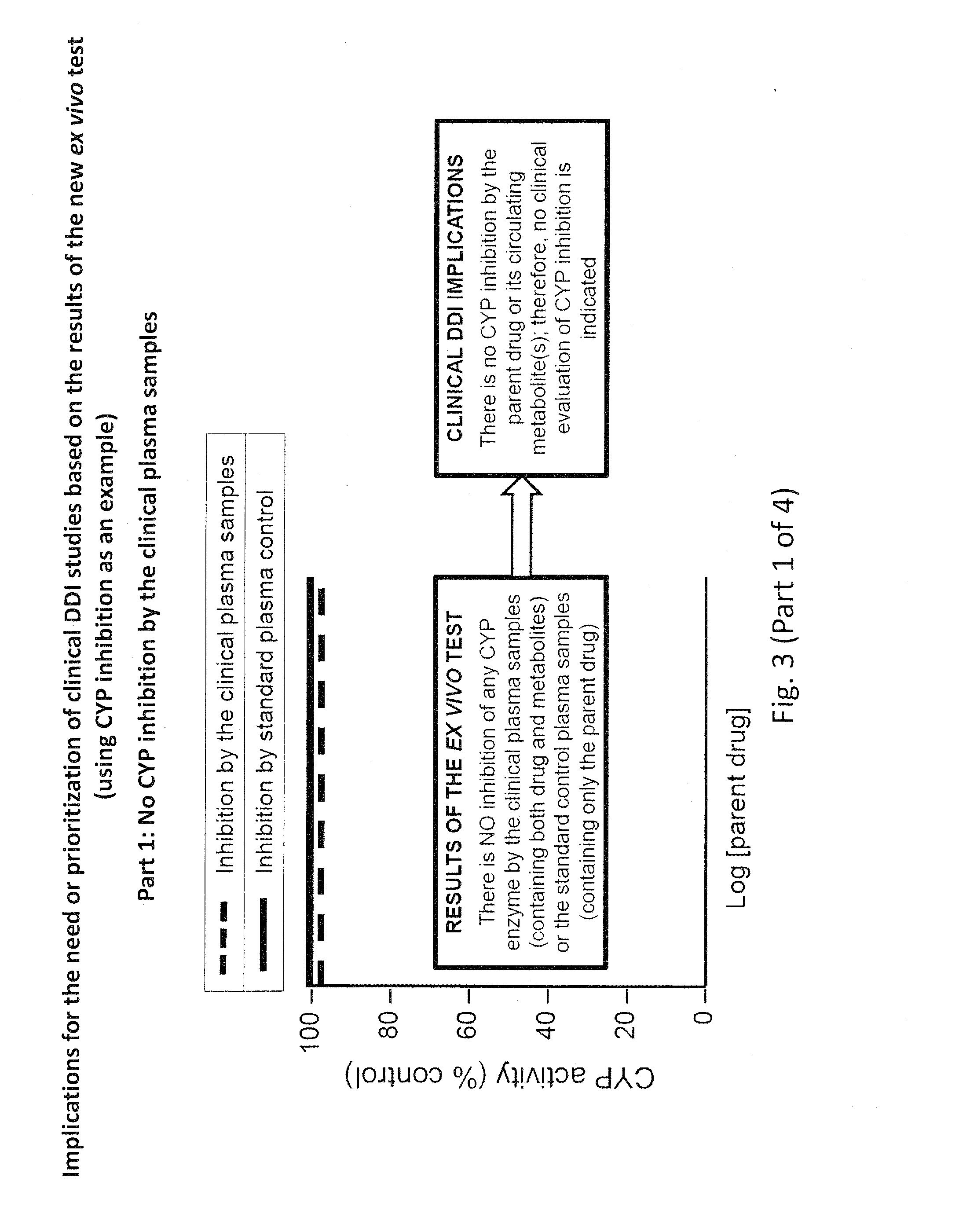
Ex vivo methods to identify circulating drug metabolites with drug interaction potential
InactiveUS20130330737A1Microbiological testing/measurementBiological testingMetaboliteDrug interaction
Ex vivo methods of detecting and analyzing circulating drug metabolites with drug interaction potential are provided. The methods include the use of clinical plasma samples from subjects who have been administered an investigational drug in vivo. The clinical plasma samples will contain both the parent drug and associated metabolites. A control plasma sample spiked directly with the drug of interest can be used as a standard reference. The plasma samples can be applied to an in vitro test system to evaluate the changes in activity or expression of drug-metabolizing enzymes and / or drug transporters in the test systems to determine circulating drug metabolites with drug interaction potential. By comparing the clinical plasma sample to the drug-spiked control, the inhibitory and / or inducing effects on drug-metabolizing enzymes and / or drug transporters can be correctly attributed to the parent drug or its associated metabolites.
Owner:XPD CONSULTING
Prediction of potential drug-drug interactions using gene expression profiling of drug transporters, cytochrome p450s and nuclear x receptors
InactiveUS20100304984A1Sugar derivativesMicrobiological testing/measurementDrug-drug interactionCytochrome P450
The invention provides materials and methods for detecting the expression of genes encoding cytochrome p450, nuclear X receptors, phase H transferases, and solute carrier family uptake pumps. The materials include sets of primers, PCR amplicons and arrays. The methods of the invention include hybridization assays. Kits and assays for the detection of the expression of the genes are also provided by the invention. In addition, the invention provides the use of the materials and methods of the invention in drug screening assays.
Owner:NOAB BIODISCOVERIES
NOVEL HEPATOCYTE-LIKE CELLS AND HEPATOBLAST-LIKE CELLS DERIVED FROM hBS CELLS
InactiveUS20110250686A1Avoid accumulationLow yieldHepatocytesAntiviralsDrug metabolismIn vitro study
Owner:CELLARTIS AB (SE)
Inhibitors of ABC drug transporters at the blood-brain barrier
InactiveUS20070054932A1Convenient treatmentModulate activityBiocideAnimal repellantsActive agentBrain concentrations
The present invention relates to inhibitors of drug transporters of the ABC protein superfamily, particularly transporters present at the blood brain barrier. ABC transporter inhibitors identified according to the invention increase brain concentrations of CNS-active agents. Such inhibitors increase the influx into the brain and / or reduce the efflux from the brain of such CNS-active agents.
Owner:SHOENHARD GRANT L
Solid self-microemulsion microcapsule containing astaxanthin and quercetin, preparation method and application thereof
InactiveCN111264860AImprove bioavailabilityImprove solubilityOrganic active ingredientsSenses disorderAstaxanthinMetabolic enzymes
The invention provides a solid self-microemulsion microcapsule containing astaxanthin and quercetin, a preparation method and application thereof. The self-microemulsion microcapsule comprises astaxanthin, quercetin, an oil phase, an emulsifier, a co-emulsifier and a solid adsorbent. According to the invention, starting from the design angle of a drug-loaded delivery system, astaxanthin and quercetin are combined to prepare a solid self-microemulsion microcapsule system, so that the traditional single carrying system of astaxanthin is broken through; the quercetin has high safety, and can inhibit the excretion effect of P-gp on medicine, so that the activity of drug metabolic enzyme CYP3A4 and drug transporter P-glycoprotein is inhibited, the bioavailability is improved, the solubility ofthe astaxanthin is improved, the quercetin and the astaxanthin can be compounded to play a role, the stability can be improved, and the oxidation resistance of the astaxanthin is not influenced; and the solid self-microemulsion microcapsule has the advantages of liquid self-microemulsion and a solid preparation, so that the dissolution rate and the bioavailability of the medicine are improved.
Owner:SHANGHAI OCEAN UNIV
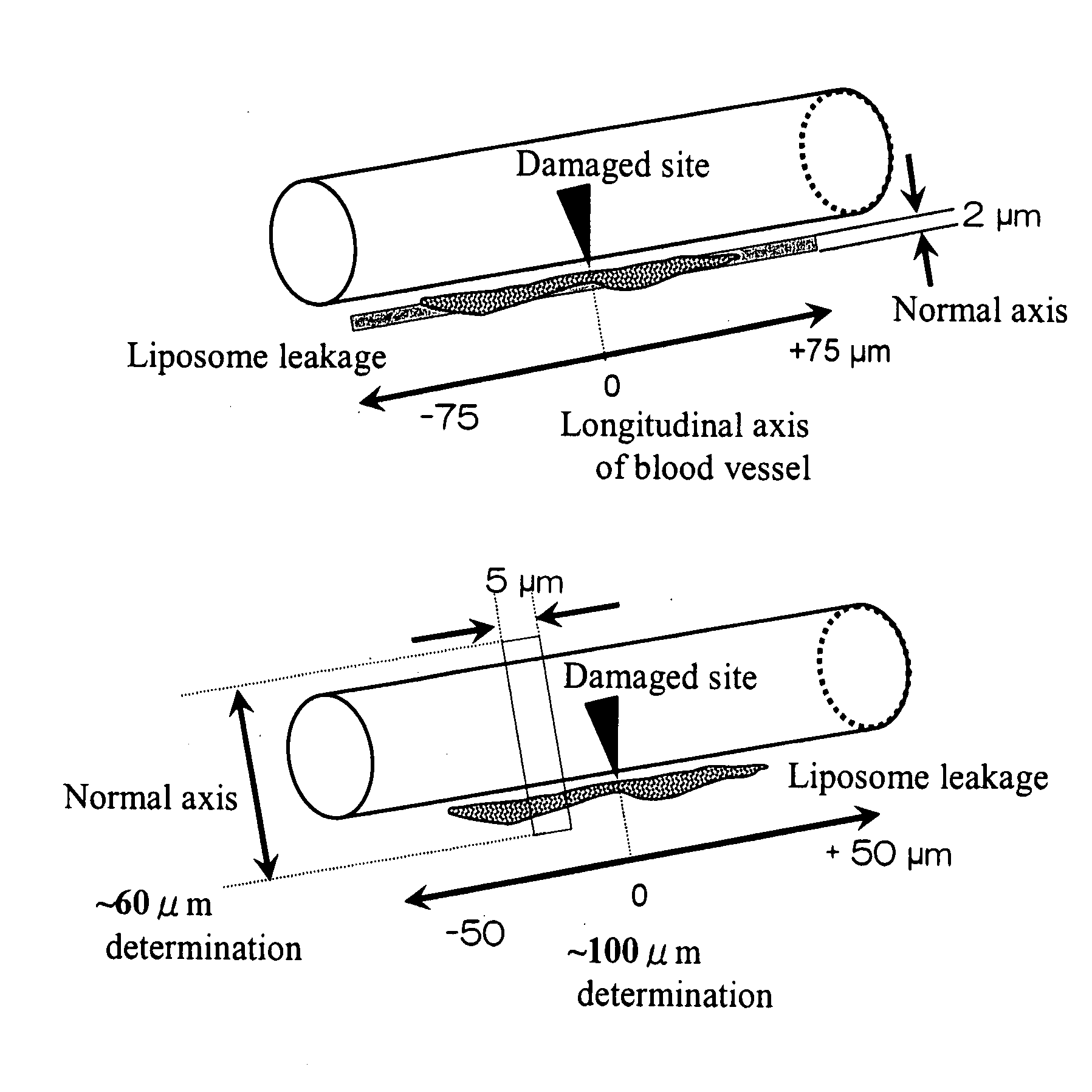
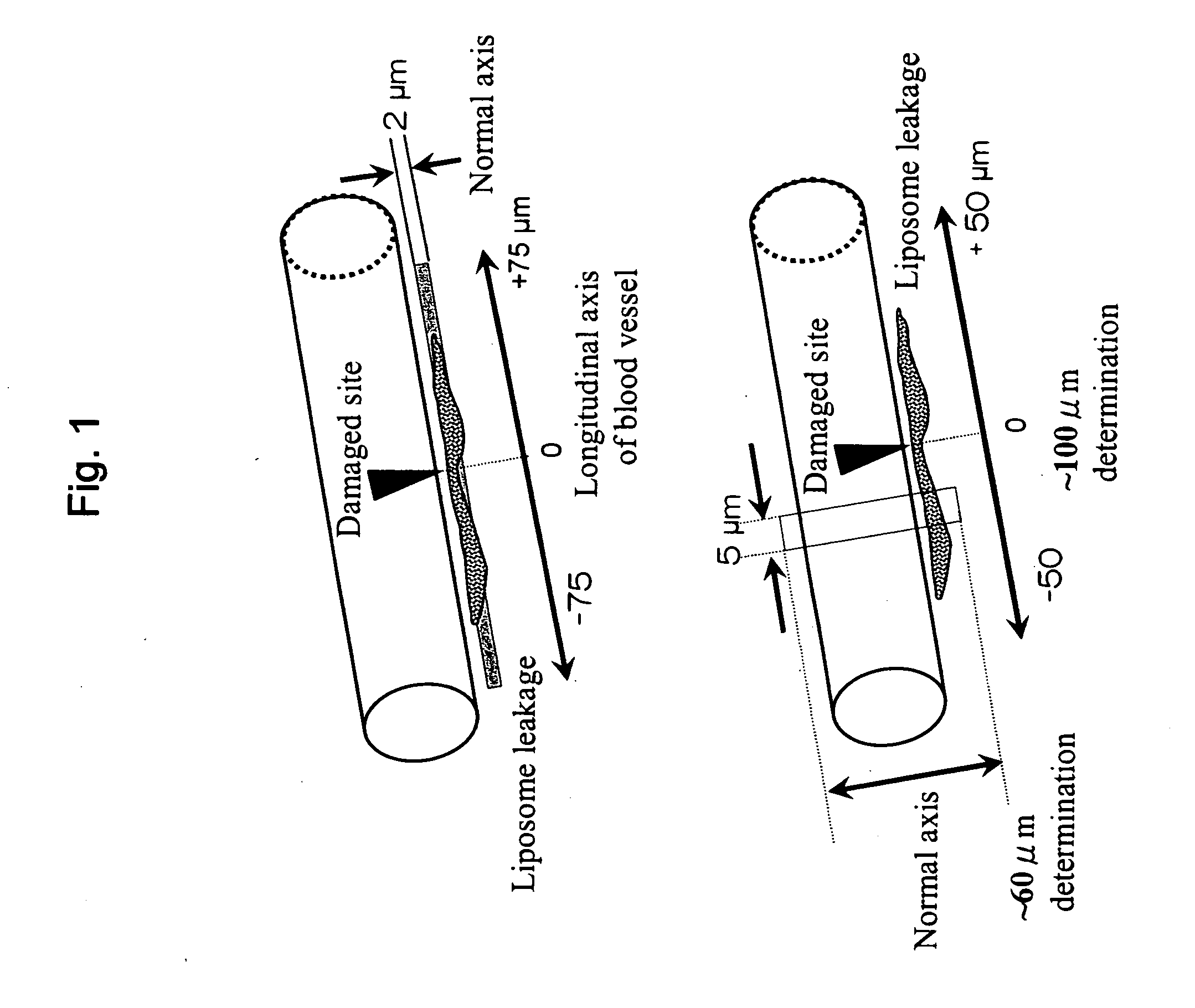
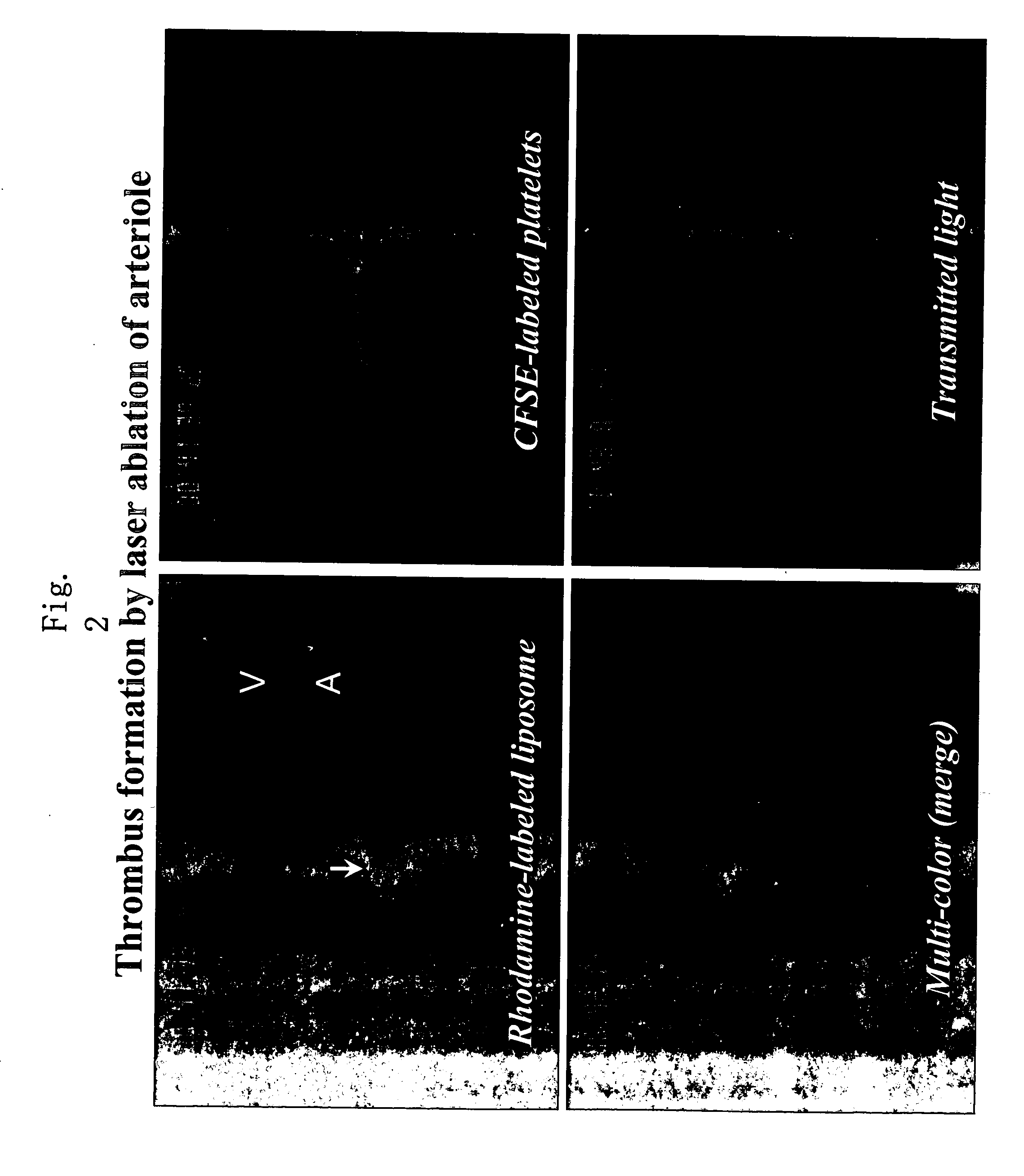
Support Accumulating In Injured Part In Vascular Channel
The present invention provides a carrier that accumulates on a damaged tissue and that functions as a drug for controlling a platelet function, a drug delivery method using the carrier and a pharmaceutical composition comprising the carrier. The present invention also provides a carrier with a non-cationic surface, a drug transporter and a pharmaceutical composition comprising the carrier.
Owner:KEIO UNIV
Diagnostic kit for early assessment of risk of adverse reactions to chemotherapy of taxanes
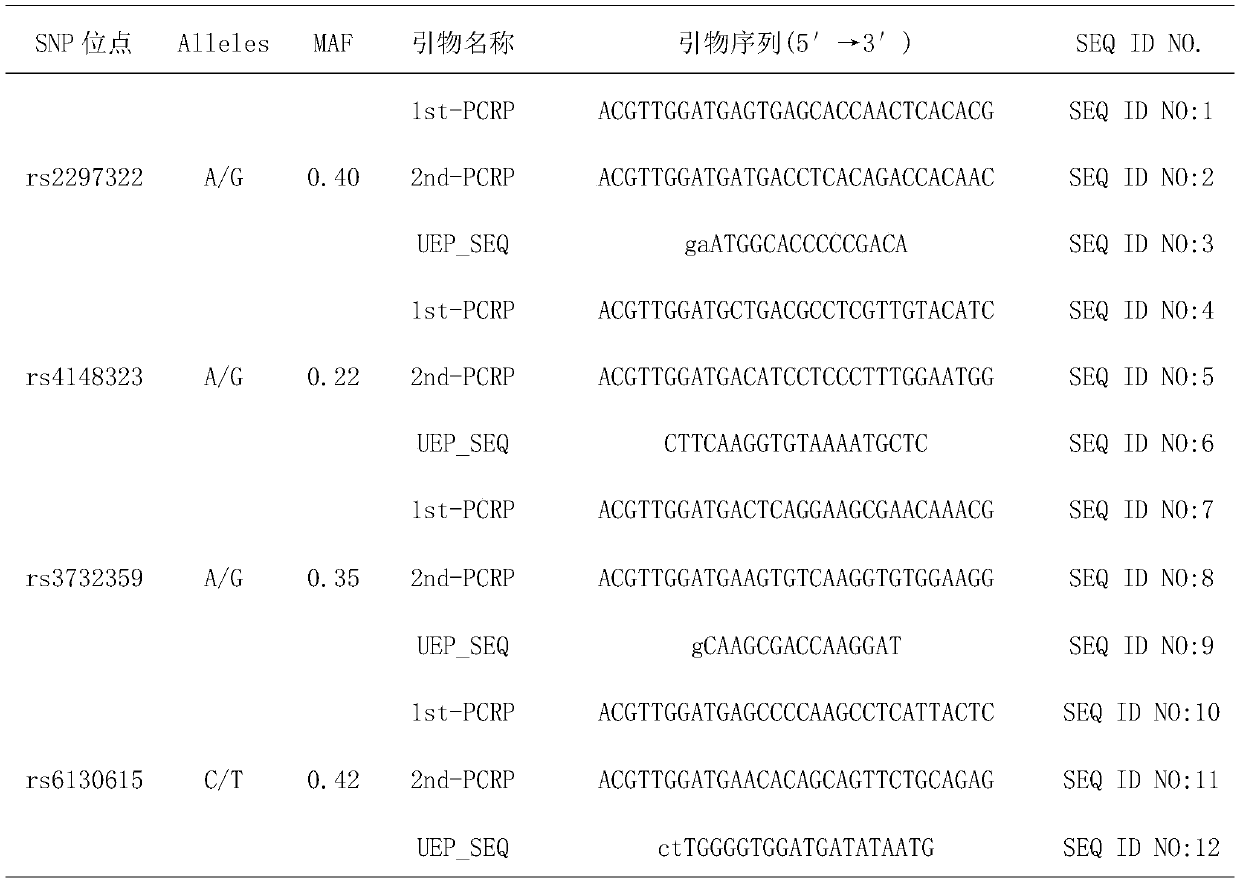
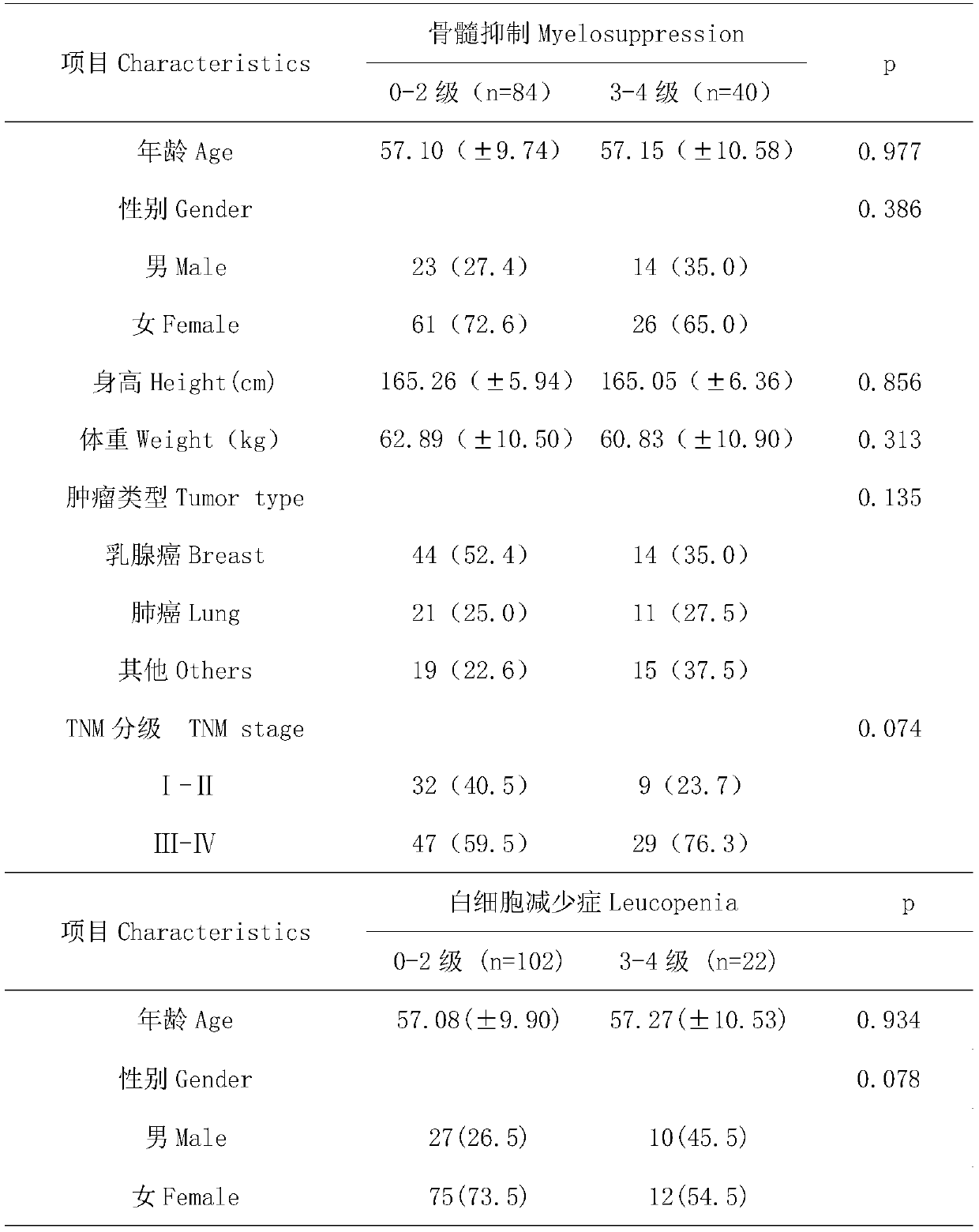
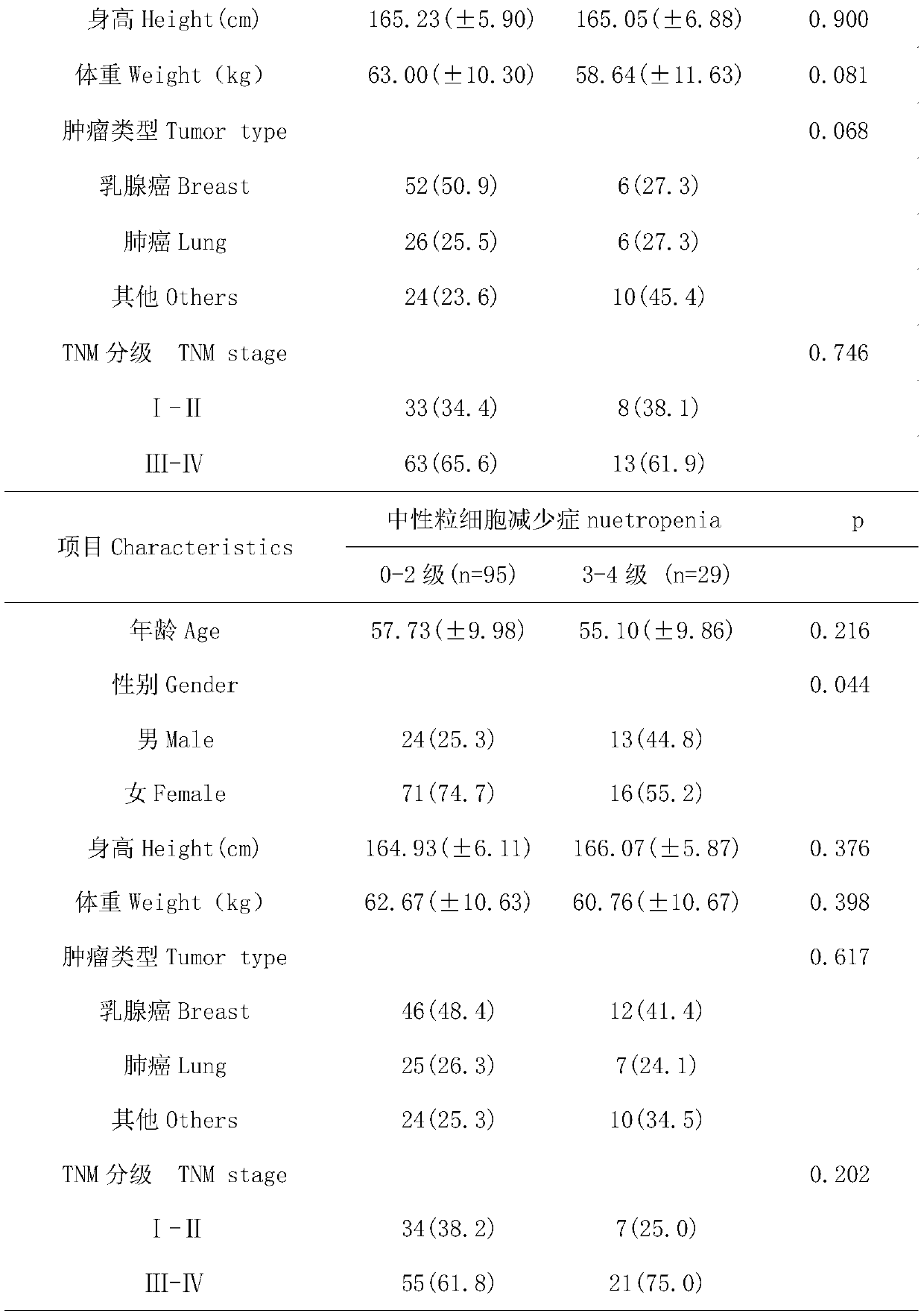
InactiveCN109750097APredict riskImproved prognosisMicrobiological testing/measurementDrug metabolismGenotype
The invention belongs to the field of genetic engineering and oncology, and discloses a diagnostic kit for early assessment of the risk of adverse reactions of chemotherapy of taxanes. The kit is usedfor predicting the risk of myelosuppression in a subject caused by chemotherapy drugs of taxanes. The kit comprises four pairs of SNPs locus genotype specific amplification primers and four specificextension primers, and targeted detection genes include a drug transporter gene SLC15A1, a drug metabolism gene UGT1A1, a nuclear receptor gene NR1I2 and a nuclear receptor gene HNF4alpha. The detection locus used in the kit is a susceptible SNPs for myelosuppression associated with the chemotherapy drugs of the taxanes. By detecting, the occurrence risk of taxane-related myelosuppression can be effectively evaluated, and the precise administration of the taxanes can be promoted.
Owner:济南市第四人民医院
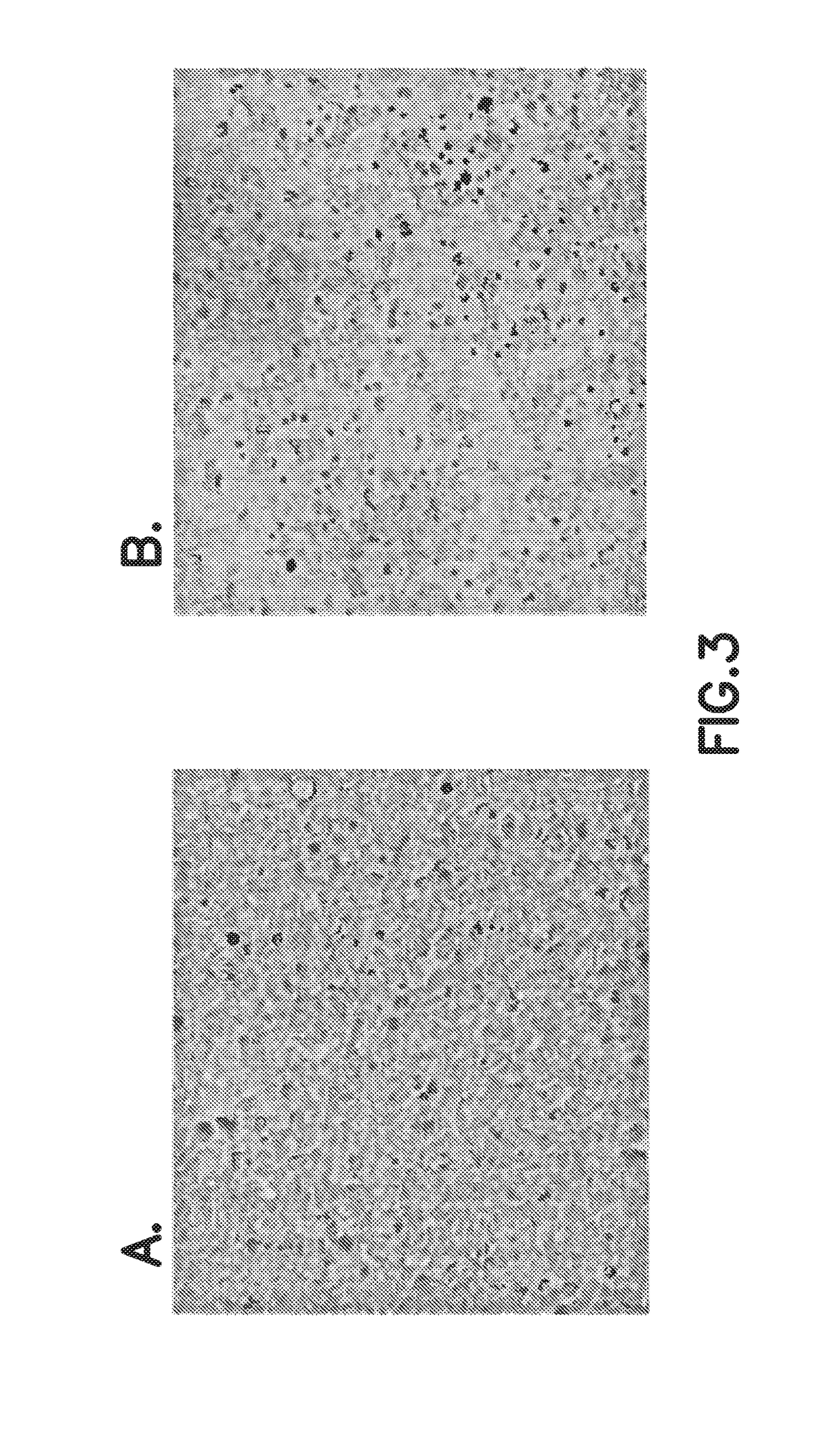
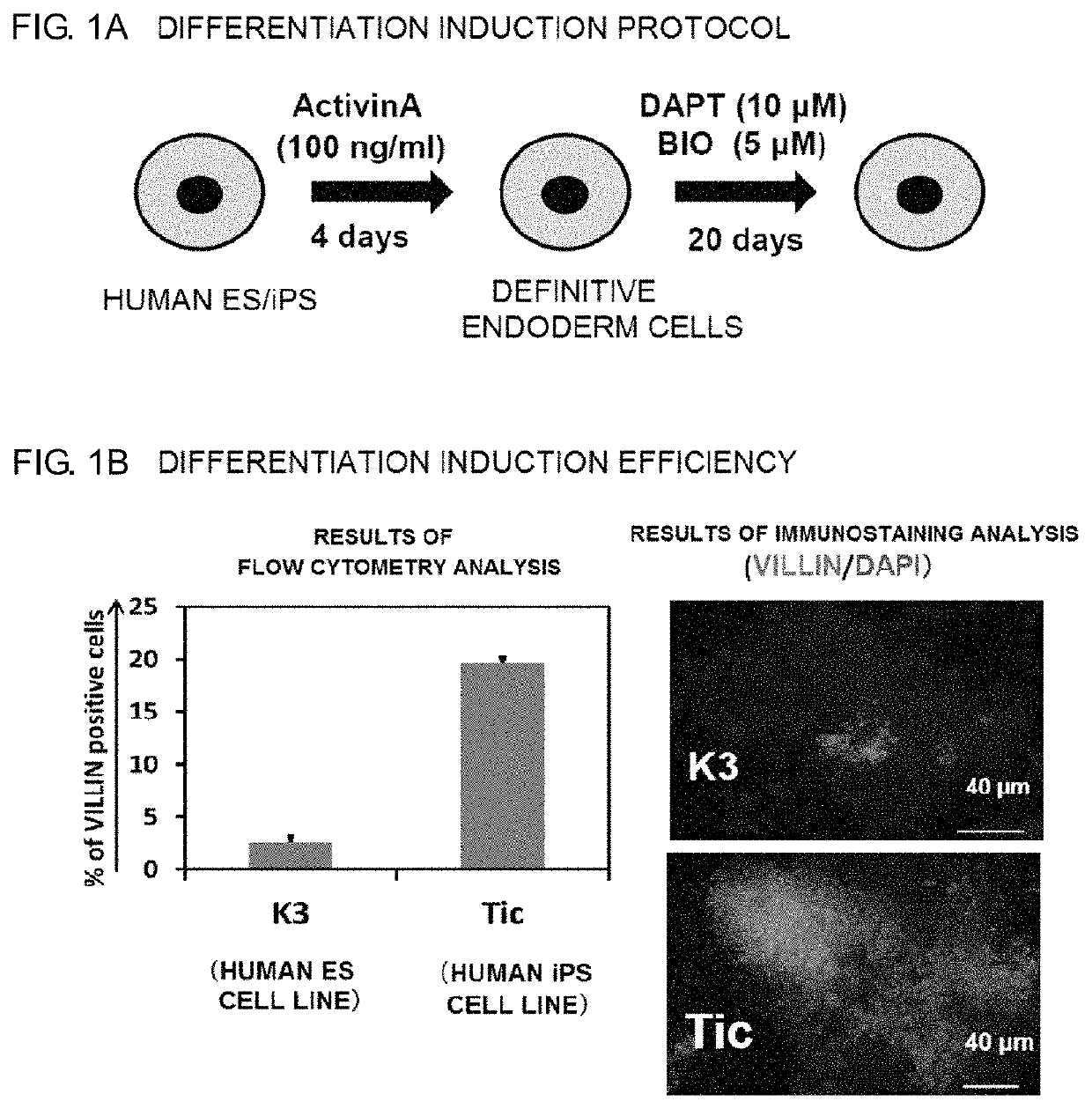
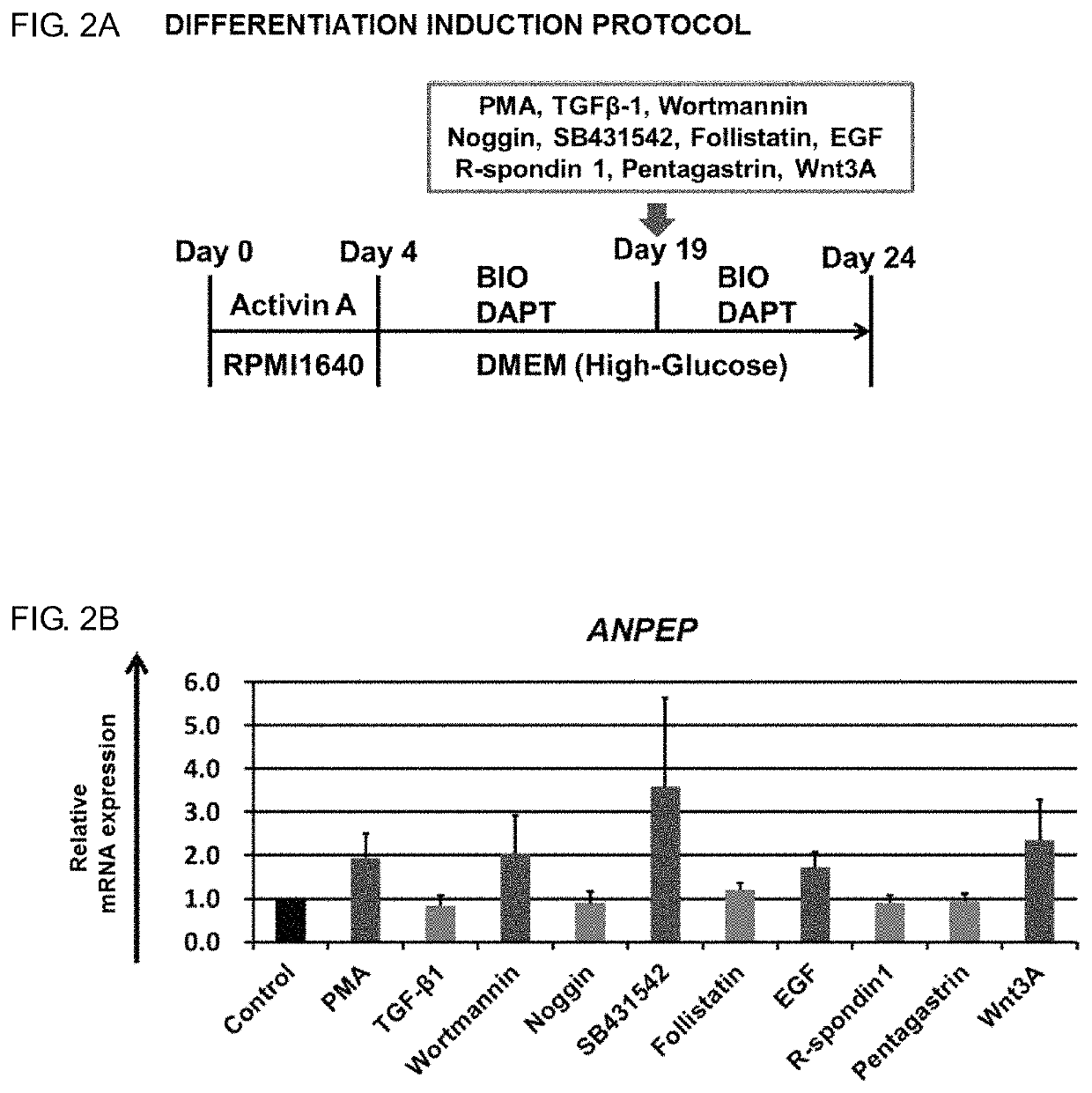
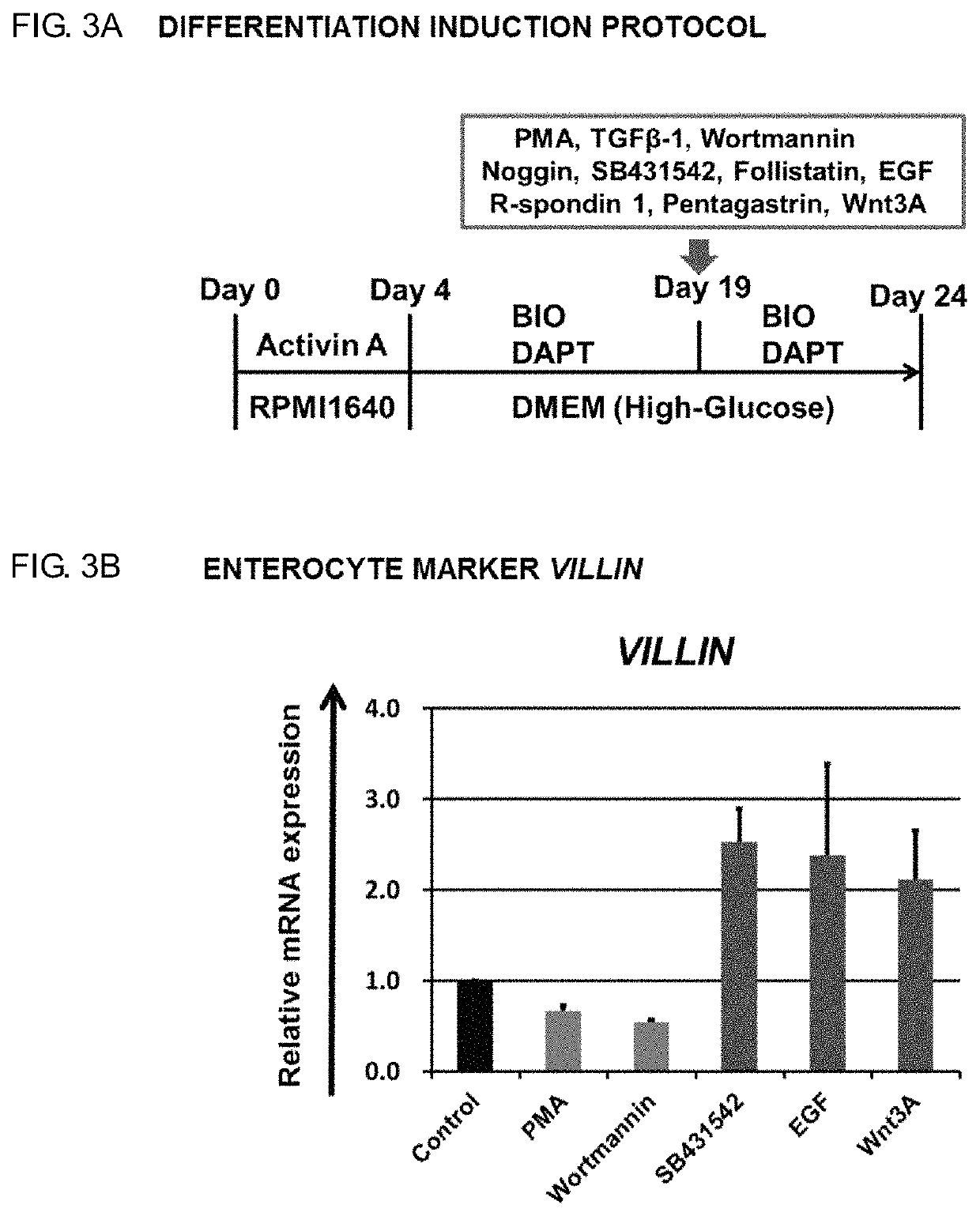
Intestinal Epithelioid Cells
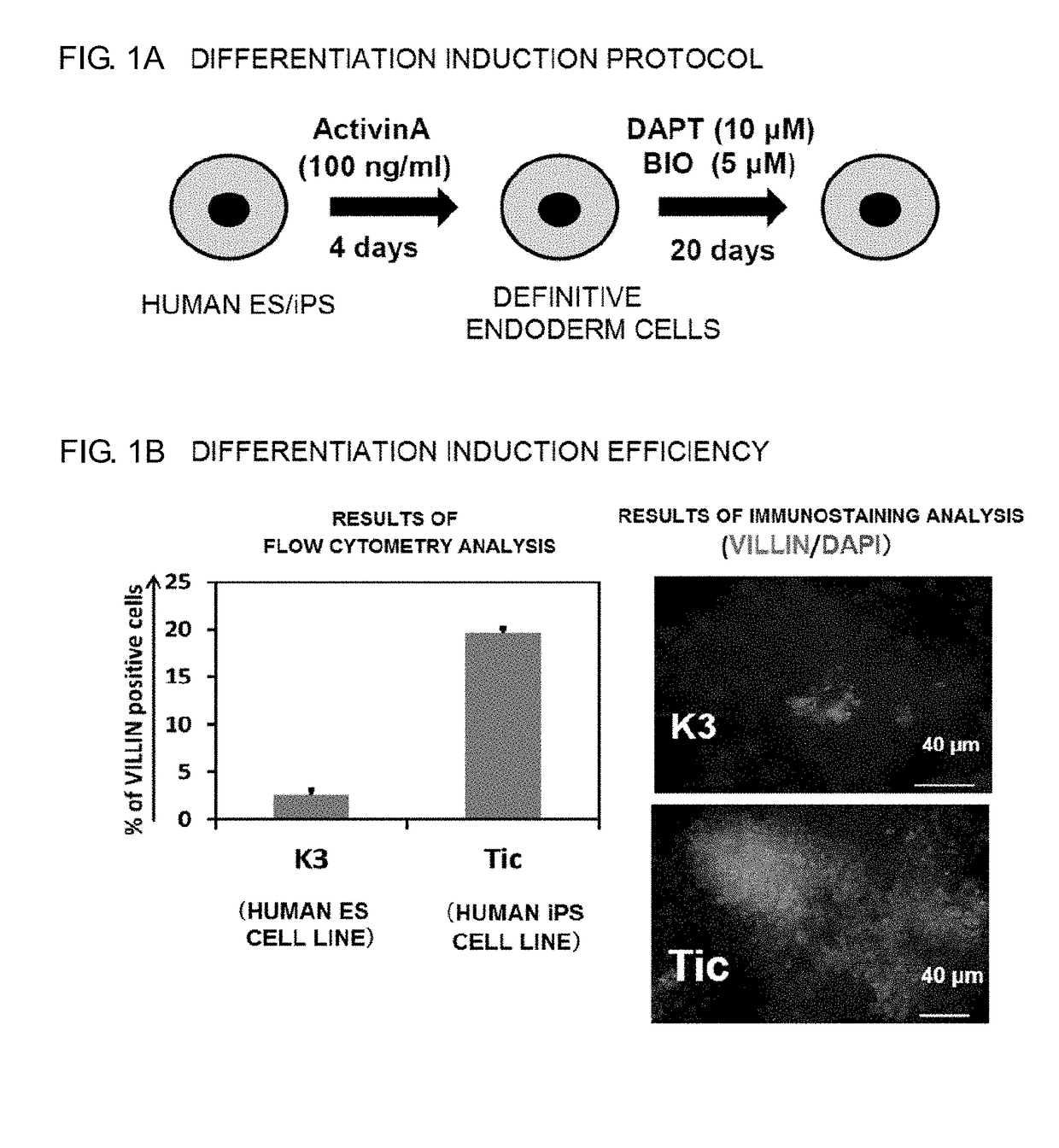
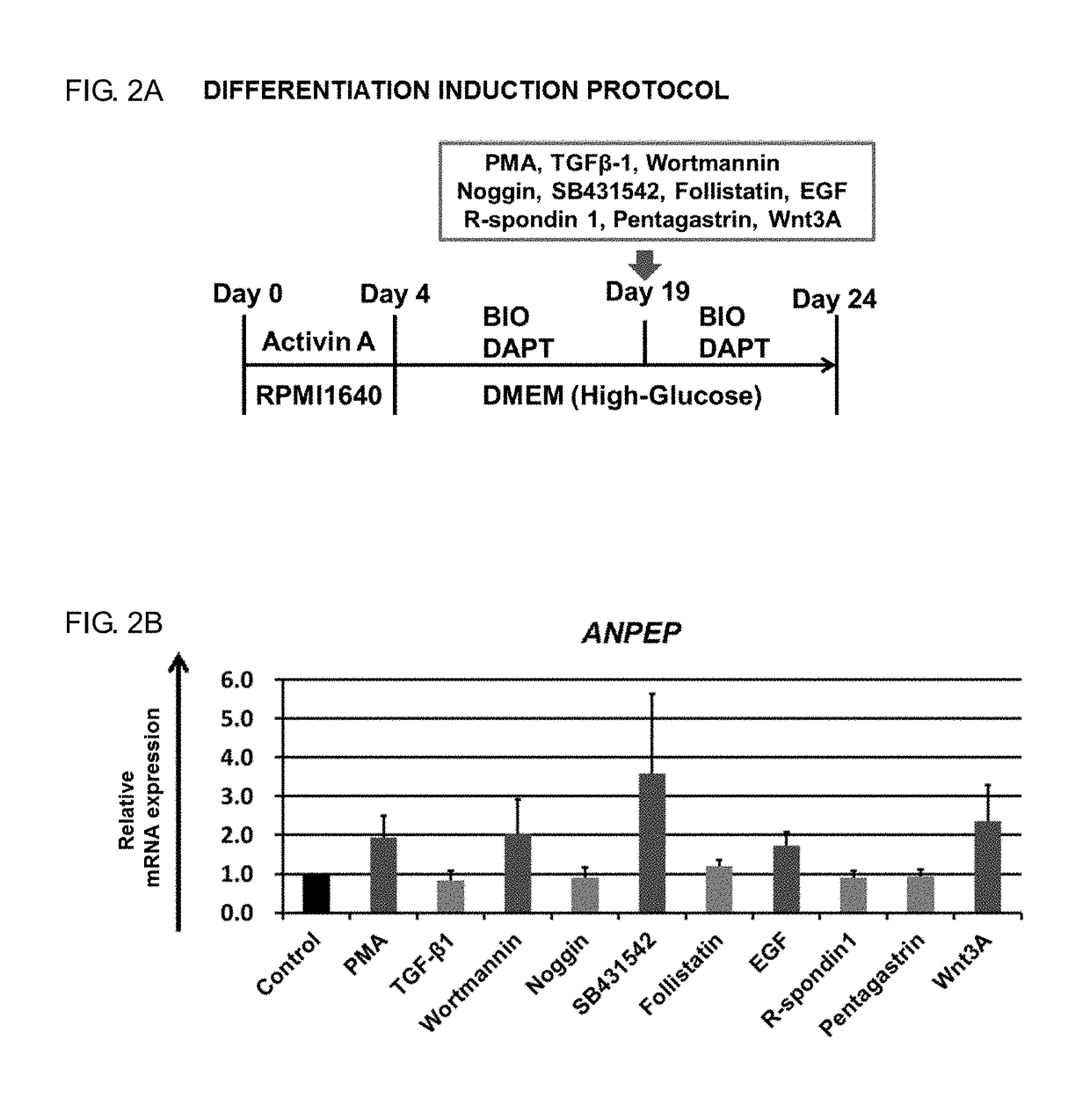
ActiveUS20180320144A1Increased gene expression levelsBeneficial level of expressionHepatocytesGastrointestinal cellsGerm layerMetabolizing enzymes
Provided is a selective method for inducing differentiation from pluripotent stem cells to enterocyte-like cells. Also provided is an excellent enterocyte-like cell expressing drug-metabolizing enzymes and drug transporters. More specifically, provided is an enterocyte-like cell having properties closer to those of primary enterocytes, which are difficult to acquire. The foregoing is achieved by adding an ALK5 inhibitor (SB431542), Wnt3a, and EGF to a culture system of definitive endoderm cells obtained by differentiation induction from pluripotent stem cells and extending a culture time. The foregoing is also achieved by introducing CDX2 gene and / or FOXA2 gene into the pluripotent stem cells or the definitive endoderm cells. The foregoing is also achieved by overlaying a basement membrane matrix on the enterocyte-like cells.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION +1
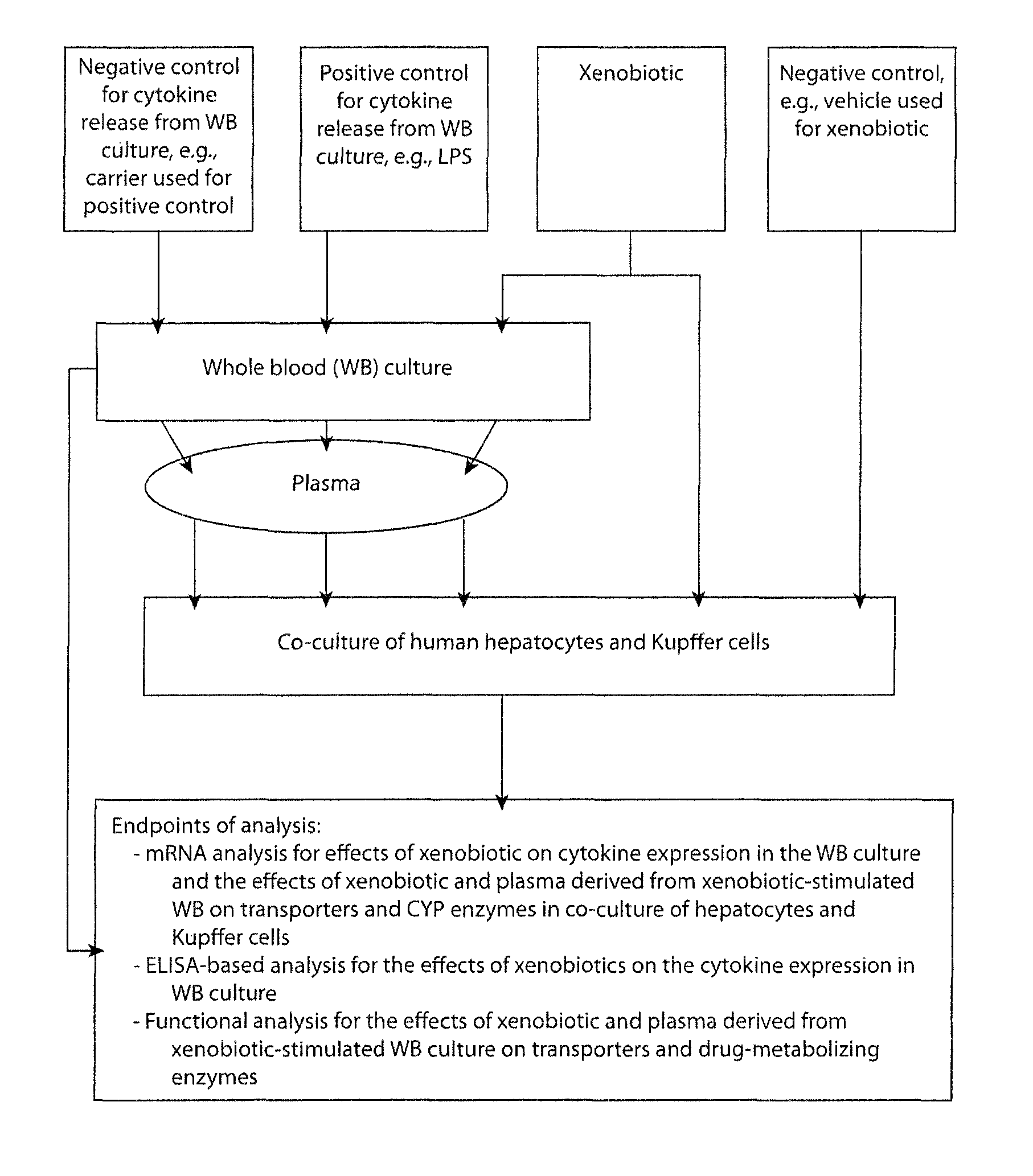
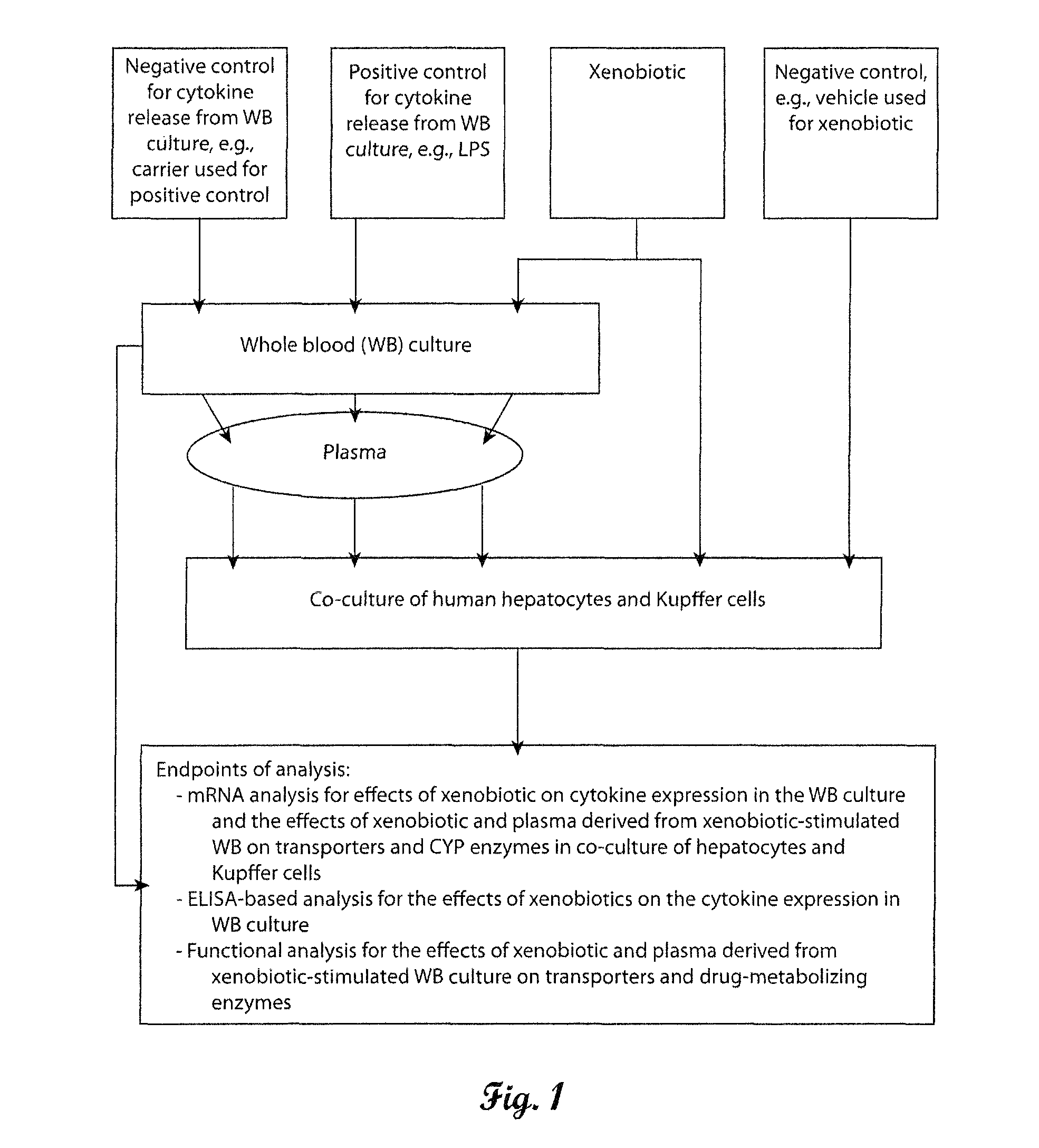
In vitro test system to evaluate xenobiotics as immune-modulators of drug transport and metabolism in human hepatocytes
Owner:XENOTECH CALIFORNIA
Nano gene drug transporter
The invention relates to a nano gene drug transporter, in particular to a nano gene drug transporter which has biodegradability and good biocompatibility, has a unique loading mode, carries unique fluorescence performance and can be used for microimaging technology and visibility research. According to the invention, a preparation method of growth type spherical calcium phosphate on a crystal nucleus is firstly designed, and then carbon quantum dots are introduced as the crystal nucleus, so that the obtained carrier obtains fluorescence performance; and the spherical calcium phosphate nano gene carrier drug carrier is prepared by taking the gene and the drug as crystal nucleuses. In the synthesized calcium phosphate nano gene drug carrier, the crystal nucleus comprises quantum dots, carbon quantum dots, drugs, nucleic acid and the like; the gene or the drug can serve as a crystal nucleus and can be loaded on the crystal nucleus or outside the spherical calcium phosphate, and loading modes comprise physical adsorption, covalent interaction, ionic bond interaction and the like.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Consumable cryopreserved cells transiently overexpressing gene(s) encoding drug transporter protein(s) and/or drug metabolizing enzyme(s)
ActiveUS9822160B2Reduces investment of time and resourceEasy to storeDead animal preservationOxidoreductasesCryopreserved CellMetabolizing enzymes
The present invention discloses cryopreserved recombinant cells for screening drug candidates that transiently overexpress one or more drug transporter proteins and / or drug metabolizing enzymes. Advantageously, such cells provide a cost-efficient consumable product that streamlines the process of screening whether drug candidates are substrates or inhibitors of drug transporter proteins and / or drug metabolizing enzymes.
Owner:DISCOVERY LIFE SCI LLC
Consumable cryopreserved cells transiently overexpressing gene(s) encoding drug transporter protein(s)
ActiveUS10047136B2Reduces investment of time and resourceEasy to storeDead animal preservationOxidoreductasesCryopreserved CellMetabolizing enzymes
The present invention discloses cryopreserved recombinant cells for screening drug candidates that transiently overexpress one or more drug transporter proteins and / or drug metabolizing enzymes. Advantageously, such cells provide a cost-efficient consumable product that streamlines the process of screening whether drug candidates are substrates or inhibitors of drug transporter proteins and / or drug metabolizing enzymes.
Owner:DISCOVERY LIFE SCI LLC
Intestinal epithelioid cells
ActiveUS10889805B2Increased gene expression levelsHigh expressionHepatocytesGastrointestinal cellsGerm layerMetabolic enzymes
Provided is a selective method for inducing differentiation from pluripotent stem cells to enterocyte-like cells. Also provided is an excellent enterocyte-like cell expressing drug-metabolizing enzymes and drug transporters. More specifically, provided is an enterocyte-like cell having properties closer to those of primary enterocytes, which are difficult to acquire. The foregoing is achieved by adding an ALK5 inhibitor (SB431542), Wnt3a, and EGF to a culture system of definitive endoderm cells obtained by differentiation induction from pluripotent stem cells and extending a culture time. The foregoing is also achieved by introducing CDX2 gene and / or FOXA2 gene into the pluripotent stem cells or the definitive endoderm cells. The foregoing is also achieved by overlaying a basement membrane matrix on the enterocyte-like cells.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION +1
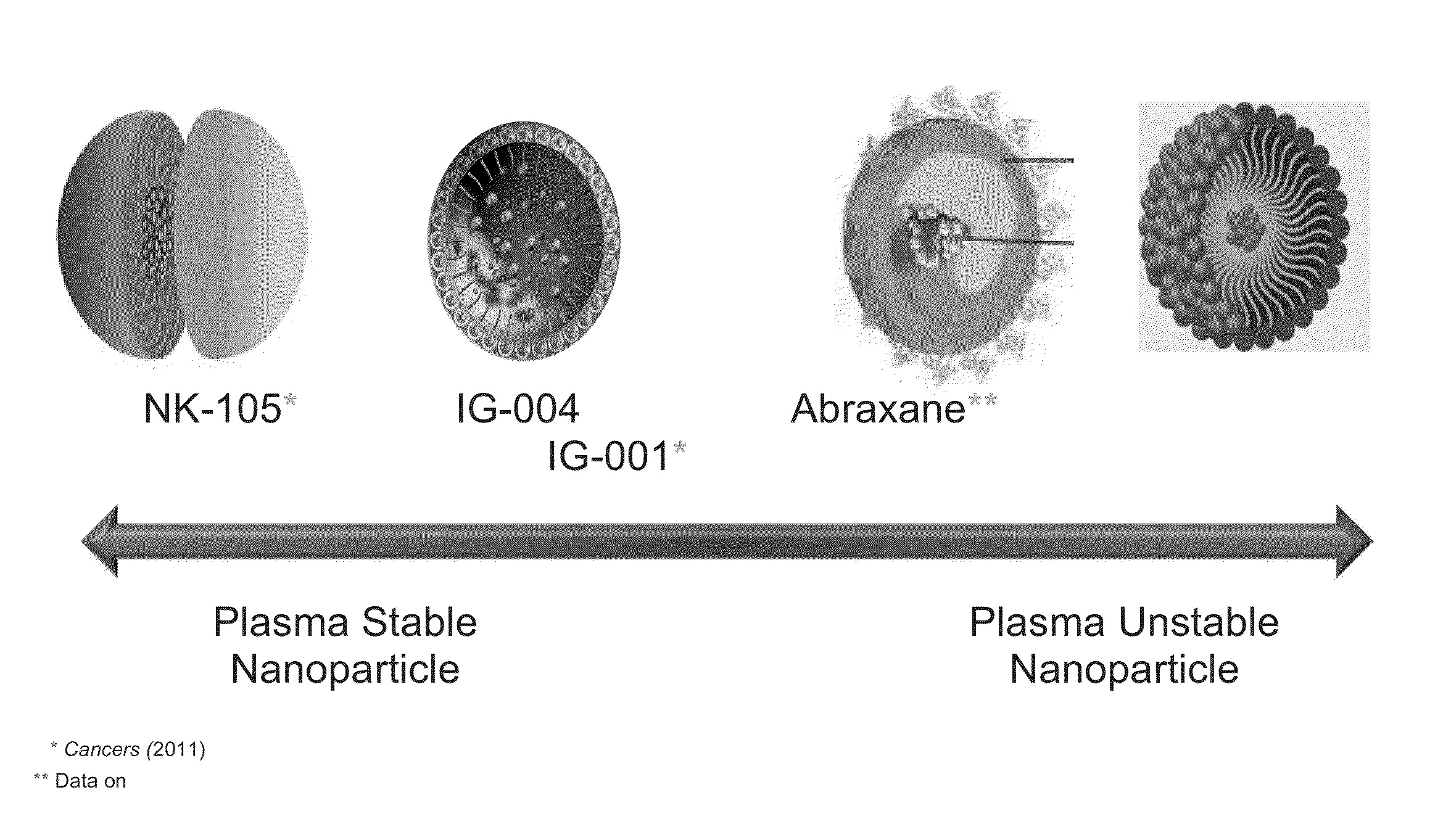
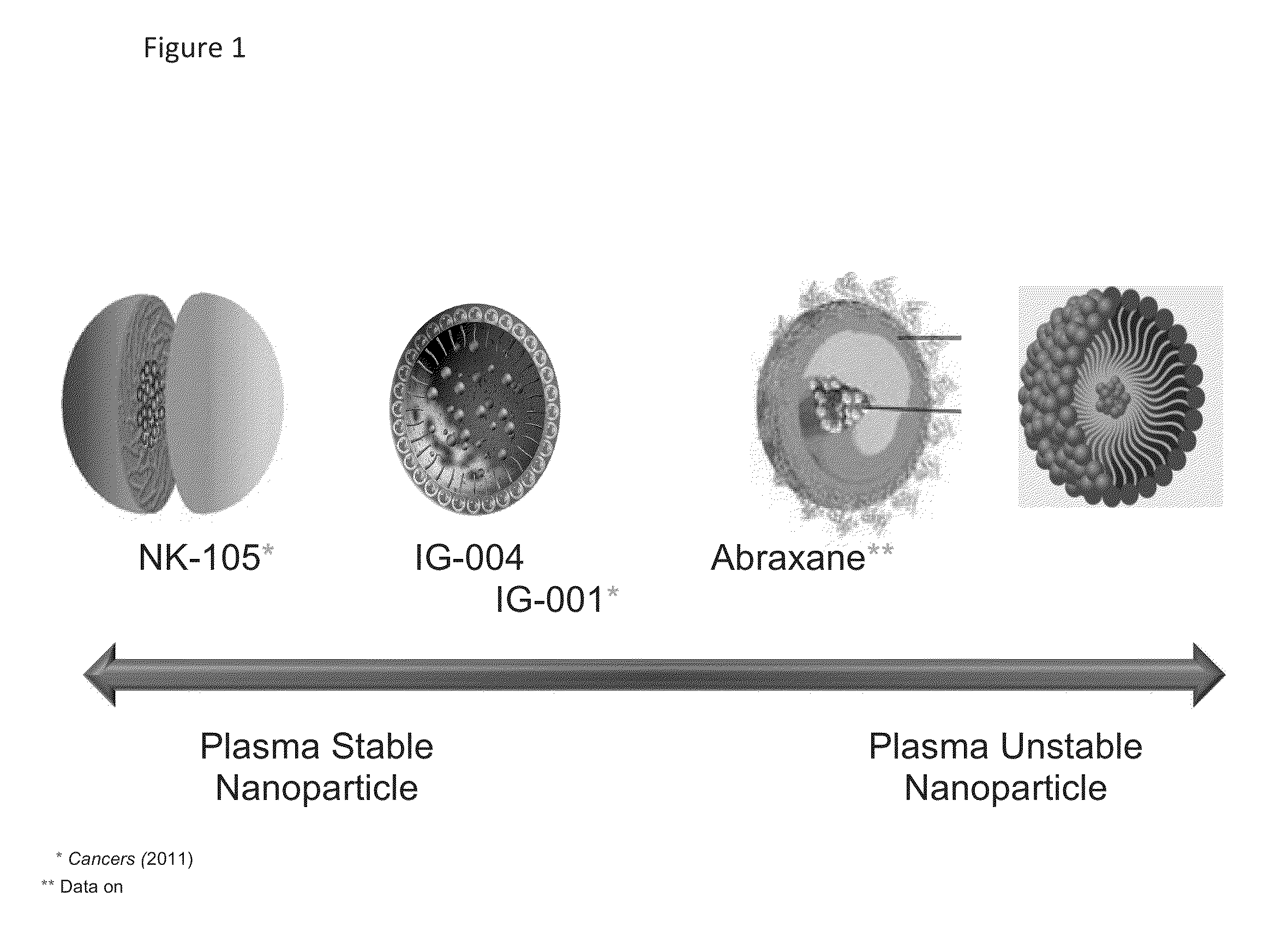
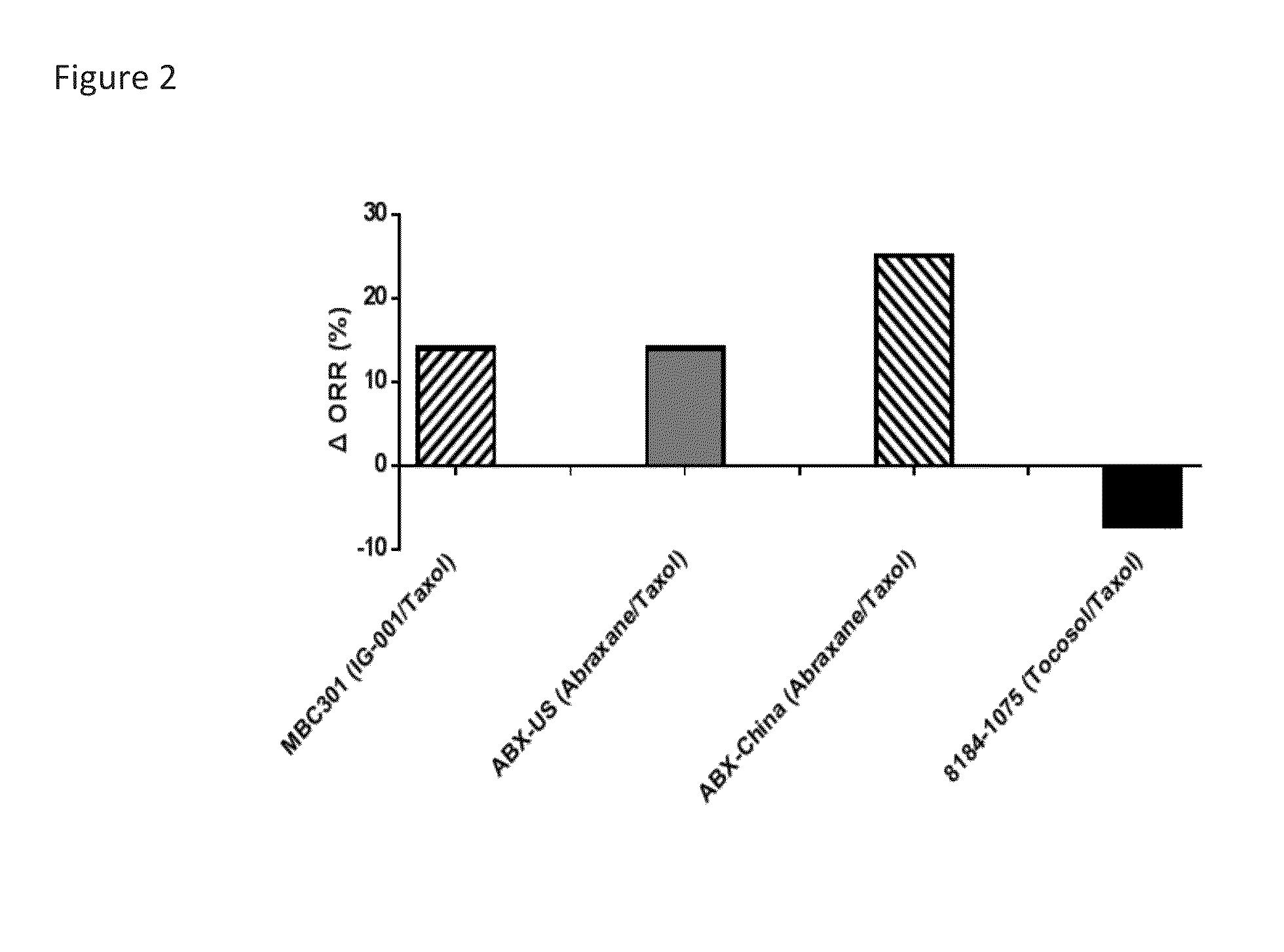
Method of Engineering Nanoparticle
The present invention relates to methods to guide the engineering of nanoparticle drugs for intravenous administration based on various pharmacokinetic parameters and other tests. The methods of the present invention have particular use in formulating nanoparticles containing cytotoxic drugs for the treatment of cancer. The guiding principles are properties which facilitate the release of drugs into the patient including unstable in plasma / blood, low AUC, low Cmax, high Vd, CMC above experimental Cmax of the drug, high tumor / plasma AUC. The present invention also provides for methods of administration and compositions which are unstable after administration to a patient so that the cytotoxic drug may bind to endogenous drug transporters and be delivered to tumors in the patient.
Owner:IGDRASOL
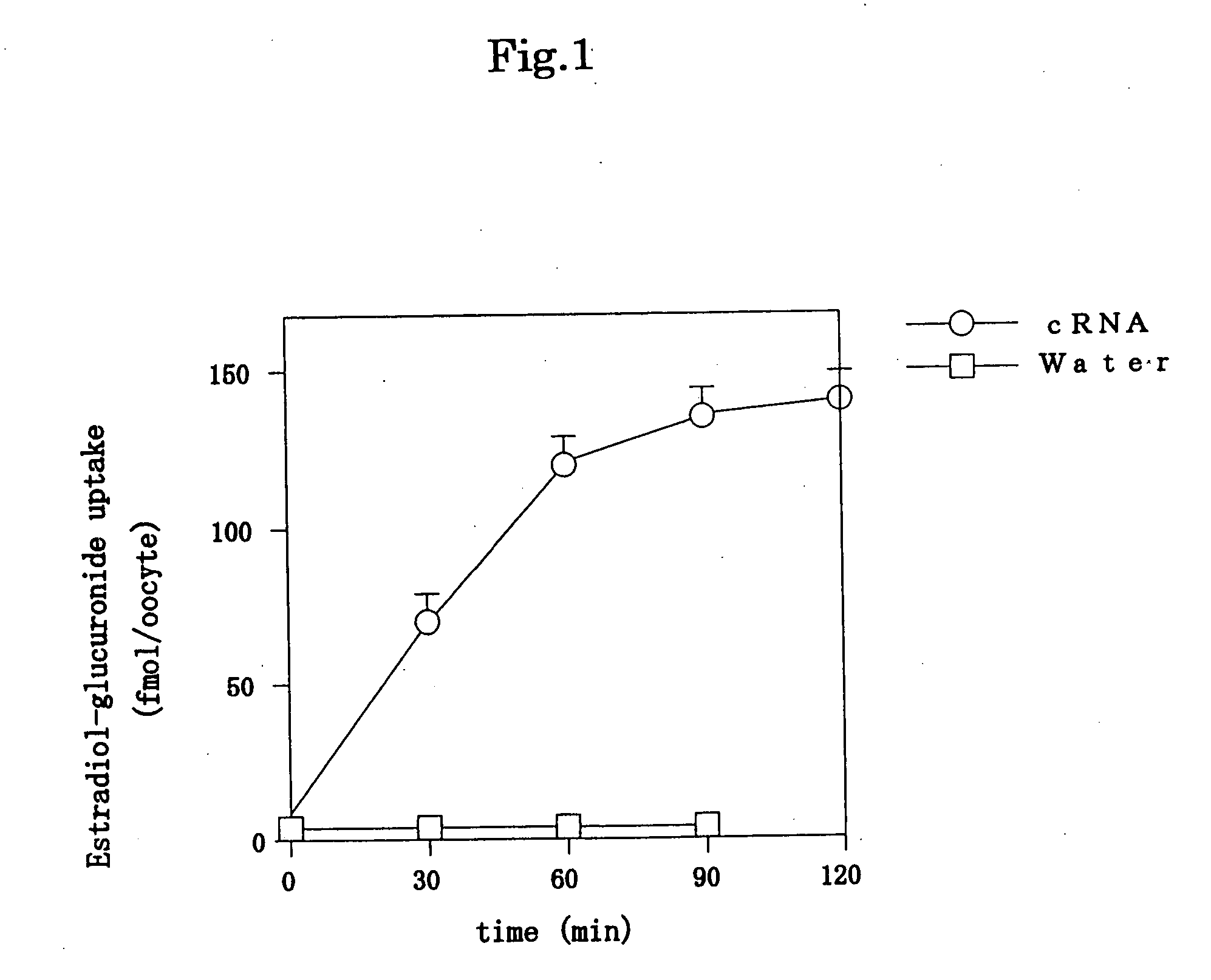
Drug transporter and use thereof
It is an object of the present invention to provide a protein having a drug transport activity, a method for screening a compound that promotes or inhibits the activity of the drug transporter, a compound obtained by the method, an antibody against the drug transporter, a pharmaceutical composition comprising the same, or the like. The protein with a drug transport activity of the present invention has an amino acid sequence represented by SEQ ID NO: 1.
Owner:IMAI MASASHI +5
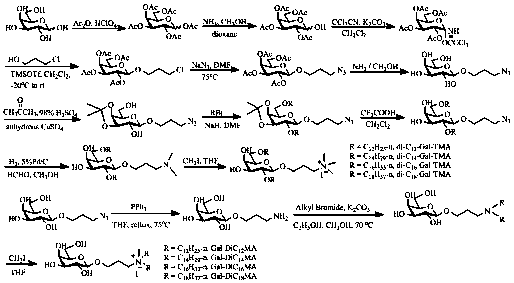
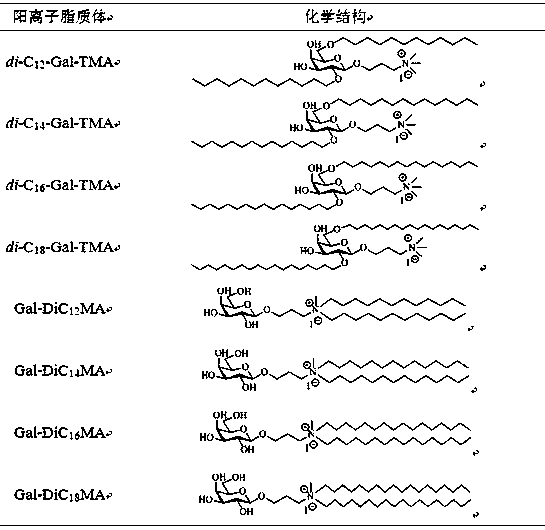
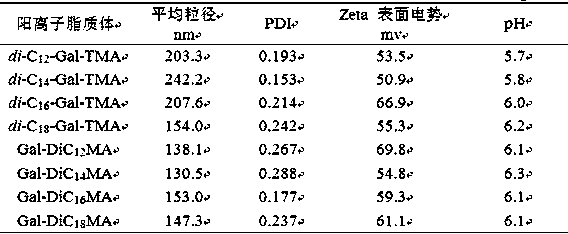
Preparation method of galactose derivative cationic liposome nanoparticles
ActiveCN106267224BEfficient preparationLow costGenetic material ingredientsPharmaceutical non-active ingredientsSurface chargesAmination
The invention discloses a method for preparing galactose derivative cationic liposome nanoparticles. Galactose as a raw material is subjected to total acetylation, 1-O-acetyl removal, trichloroacetic imine esterification, glycosylation, azidation and all acetyl removal reactions to obtain an intermediate, the intermediate is subjected to 3,4-O-isopropylidene formation, etherification, isopropylidene removal, reductive amination, quaternary ammonium salinization and reductive ammoniation, tertiary amination and quaternary ammonium salinization reactions, and two series of galactose derivative cationic liposomes with different physical structures are synthesized; after water dispersion, the corresponding cationic liposome nanoparticles are obtained, have the advantages of good stability, moderate particle size and surface charges, low preparation cost and the like, and can meet basic requirements required for nucleic acid drug transporters.
Owner:湖南远泰生物技术有限公司
RAT GENE EXPRESSION PROFILING OF DRUG TRANSPORTERS, CYTOCHROME P450s, TRANSFERASES AND NUCLEAR XENOBIOTIC RECEPTORS FOR PREDICTING DRUG EFFECTS
InactiveUS20100160176A1Predict potentialTo promote metabolismNucleotide librariesMicrobiological testing/measurementPrimary cellCytochrome p450 enzyme
The disclosure describes materials and methods for detecting the expression of genes and generating a gene expression profile from drug-treated rat primary cells or established rat cell lines using a unique combination of rat cytochrome p450 enzyme, nuclear xenobiotic receptor, transferase and transporter gene sequences. The materials include sets of primers, PCR amplicons and arrays. The methods include hybridization assays. Assays for the detection of the expression of the genes are also provided. In addition, the disclosure provides the use of the materials and methods in drug screening assays and, specifically, the detection of potential drug-drug interaction(s).
Owner:NOAB BIODISCOVERIES
Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent
ActiveCN111467341BIncreased drug resistanceIncreased sensitivityOrganic active ingredientsOrganic chemistryBenzoxazoleChemotherapy combinations
The present invention specifically relates to the application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor drug resistance reversal agent. In the late stage of tumor treatment, the main reason for the failure of chemotherapy regimens comes from the drug resistance of tumor cells. Existing related studies have shown that the cause of drug resistance in tumor cells is related to drug transporters. The research of the present invention shows that 4,6-di-tert-butyl-2-(3,4-dimethoxyphenyl)benzo[d]oxazole-7-ol can inhibit the transport-related proteins in tumor cells and reverse the Drug resistance can improve the sensitivity of tumor cells to anti-tumor drugs, and can be used as an adjuvant therapy for chemotherapy regimens and co-administered with other anti-tumor drugs, which has good clinical significance.
Owner:无锡享源信息科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









![Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent](https://images-eureka.patsnap.com/patent_img/769b68ae-bd7d-4d04-97c1-bcf44dce9014/BDA0002465809950000021.png)
![Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent](https://images-eureka.patsnap.com/patent_img/769b68ae-bd7d-4d04-97c1-bcf44dce9014/BDA0002465809950000031.png)
![Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent Application of 3,4-dimethoxyphenyl-benzo[d]oxazole as a tumor resistance reversal agent](https://images-eureka.patsnap.com/patent_img/769b68ae-bd7d-4d04-97c1-bcf44dce9014/BDA0002465809950000051.png)