Patents
Literature
83 results about "Caco-2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Caco-2 cell line is a continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells, developed by the Sloan-Kettering Institute for Cancer Research through research conducted by Dr. Jorgen Fogh.
Caco-2 cell model for CRISPR/CAS9-mediated drug transporter targeted knockout and method thereof
The invention discloses a caco-2 cell model for CRISPR / CAS9-mediated drug transporter targeted knockout and a method thereof. The method is used for non-diagnostic or therapeutic purposes and comprises the following steps: designing sgRNA with target specificity for P-gp, BCRP and MRP2 transporters and constructing a sgRNA expression vector, wherein a sequence of the designed sgRNA is shown as SEQID NO. 1-6 in a sequence table; respectively designing P-gp, BCRP and MRP gene single knockout and pairwise combination double knockout by utilizing CRISPR / CAS9, co-transfecting a caco-2 cell with anhCas9 plasmid and performing monoclonal expansion culture to obtain the caco-2 cell model for transporter gene targeting. The caco-2 cell model obtained by the method disclosed by the invention has the beneficial effects that the mutual interference among different transporters is effectively eliminated, and a more specific and more sensitive cell model is provided for drug transport research.
Owner:SOUTH CHINA UNIV OF TECH
1-phenylalcoxy-2-beta-phenylethyl derivatives as p-glycoprotein (p-gp) inhibitors useful in drug resistance events
The invention relates to a new class of compounds, which are 1-phenylalcoxy-2-β-phenylethyl derivatives, as P-glycoprotein (P-GP) inhibitors. These compounds are useful in drug resistance events. They have been shown able to inhibit in a dose-dependent manner Glycoprotein-P (P-gp) activity in cell lines in which the expression of said glycoprotein is very high, like Caco-2 (human colon cancer) cells and MCF7 / Adr (adriamycin-resistant human breast carcinoma) cells. The invention also relates to methods of production and the utilization of such compounds as medicaments useful in the treatment of states linked to the difficulty for some drugs to cross the blood-brain barrier (BBB) and generally within the context of the problems of drug resistance induced by chemotherapy agents.
Owner:UNIV DEGLI STUDI DI BARI 60 +1
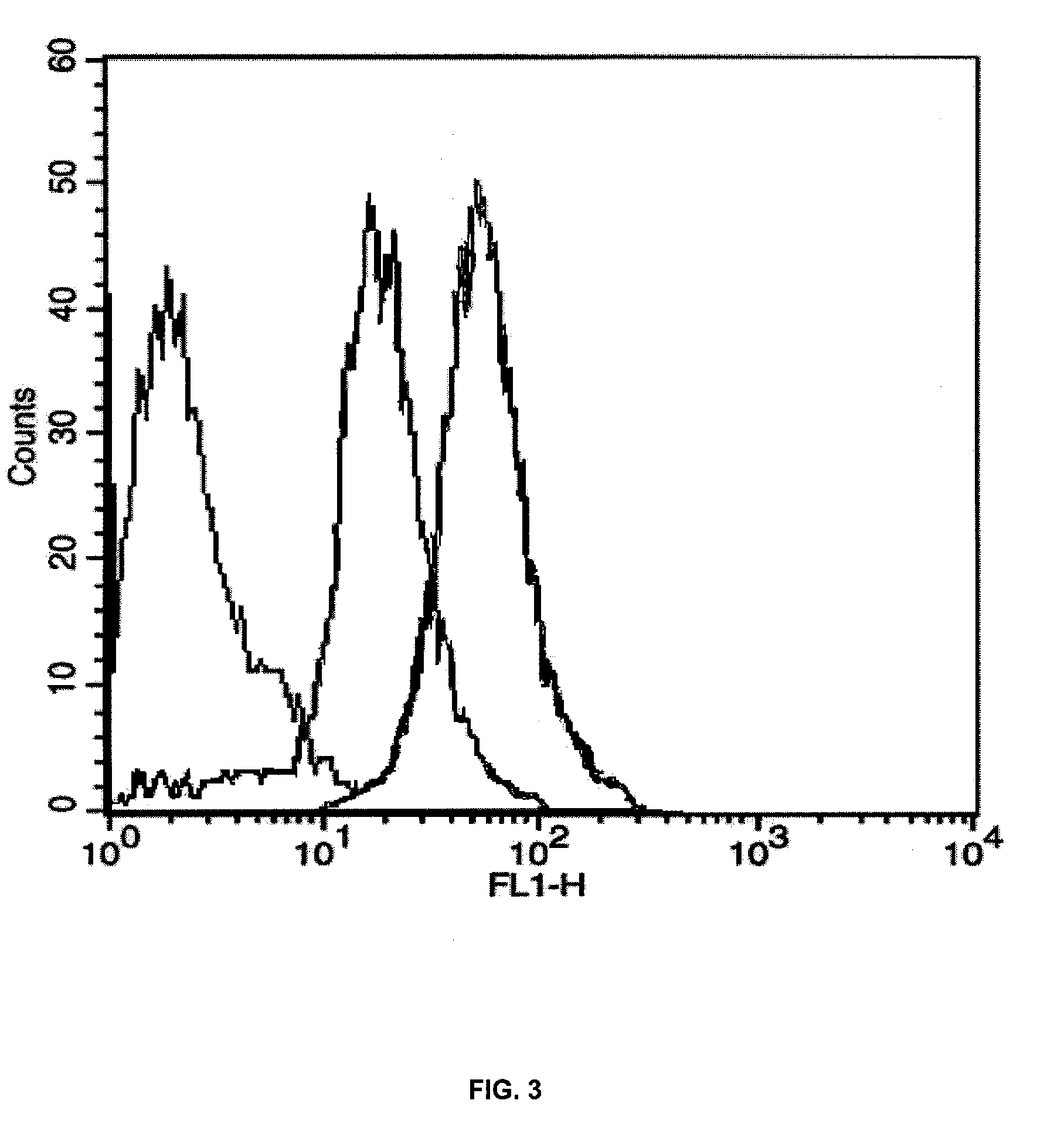
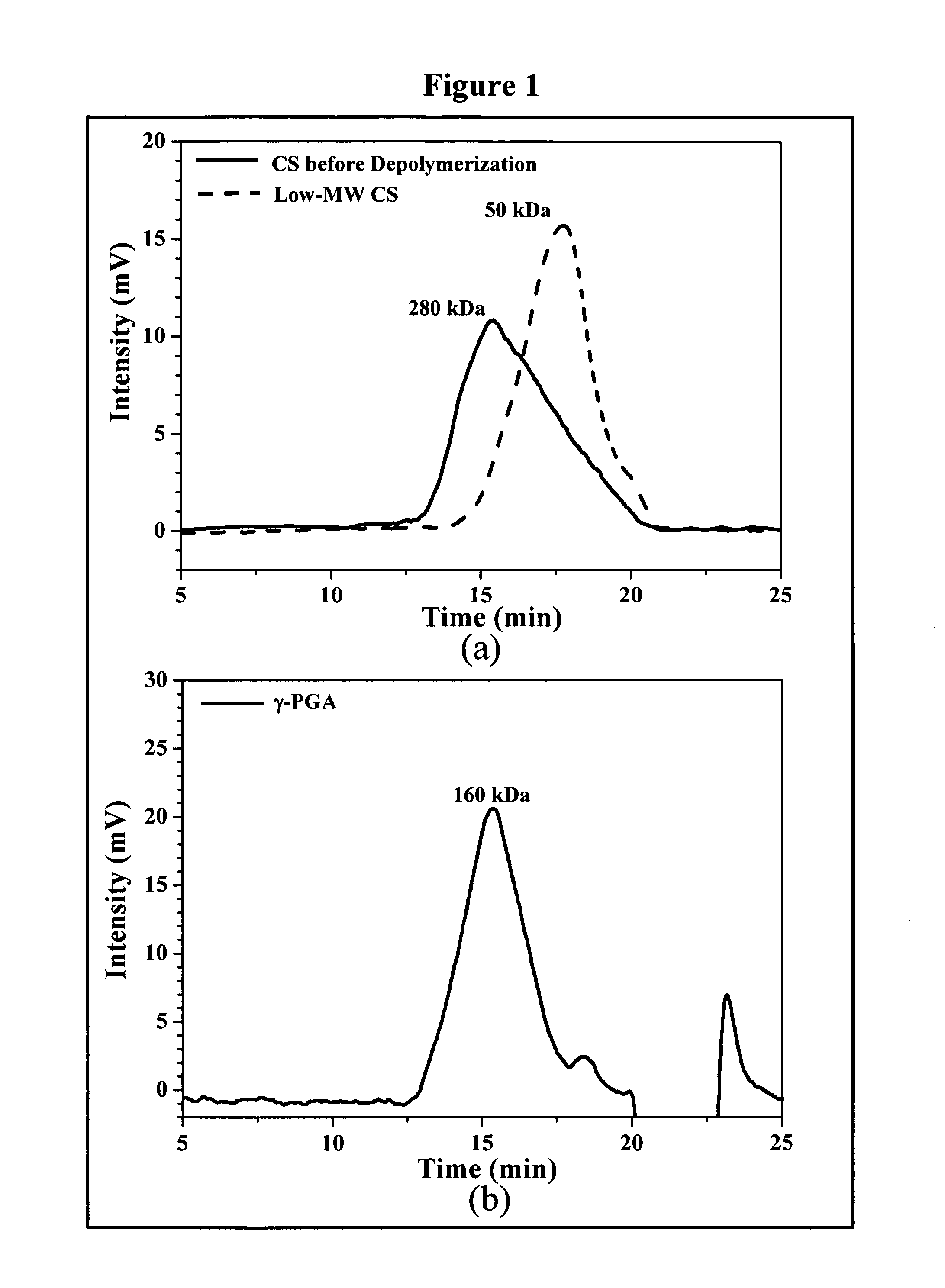
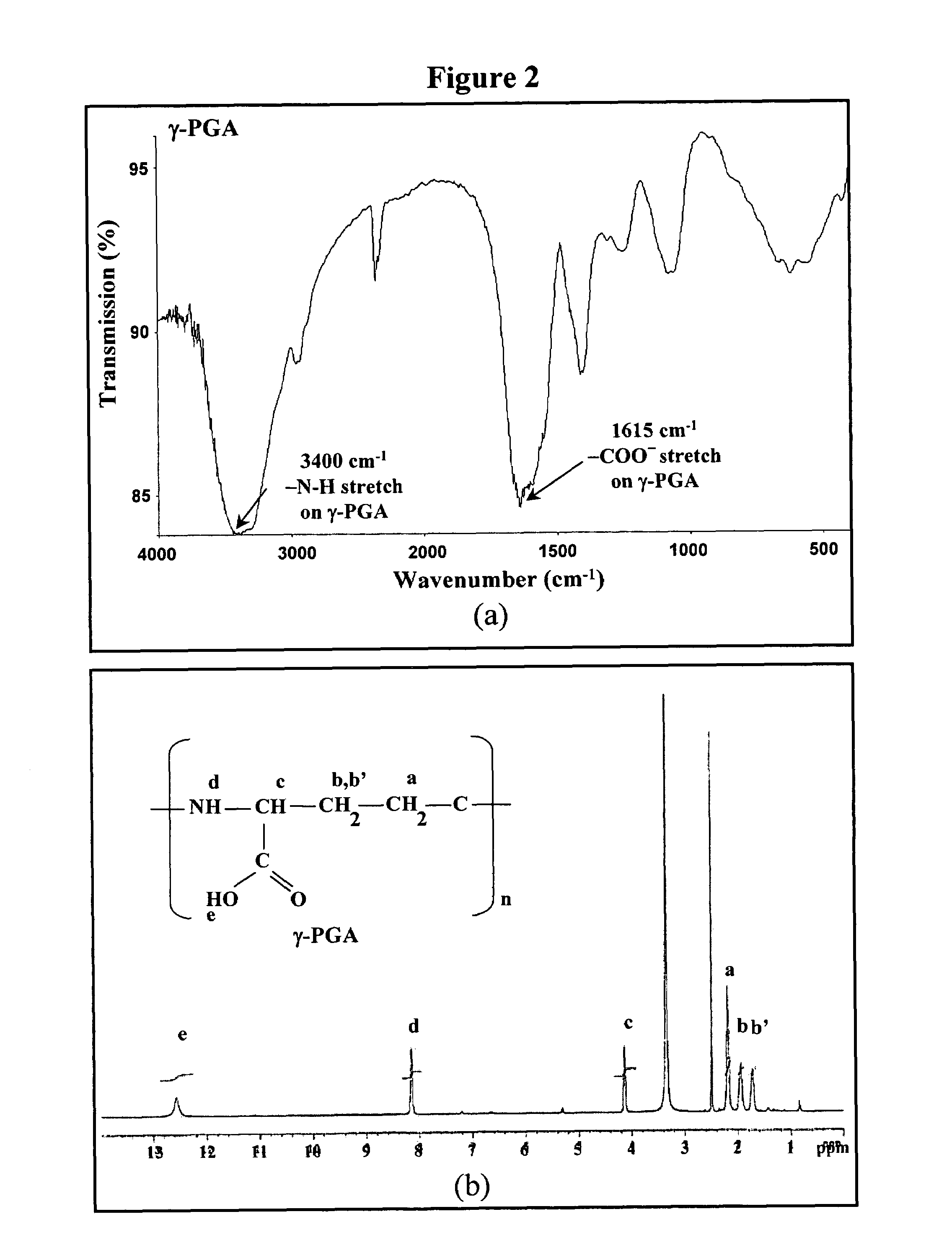
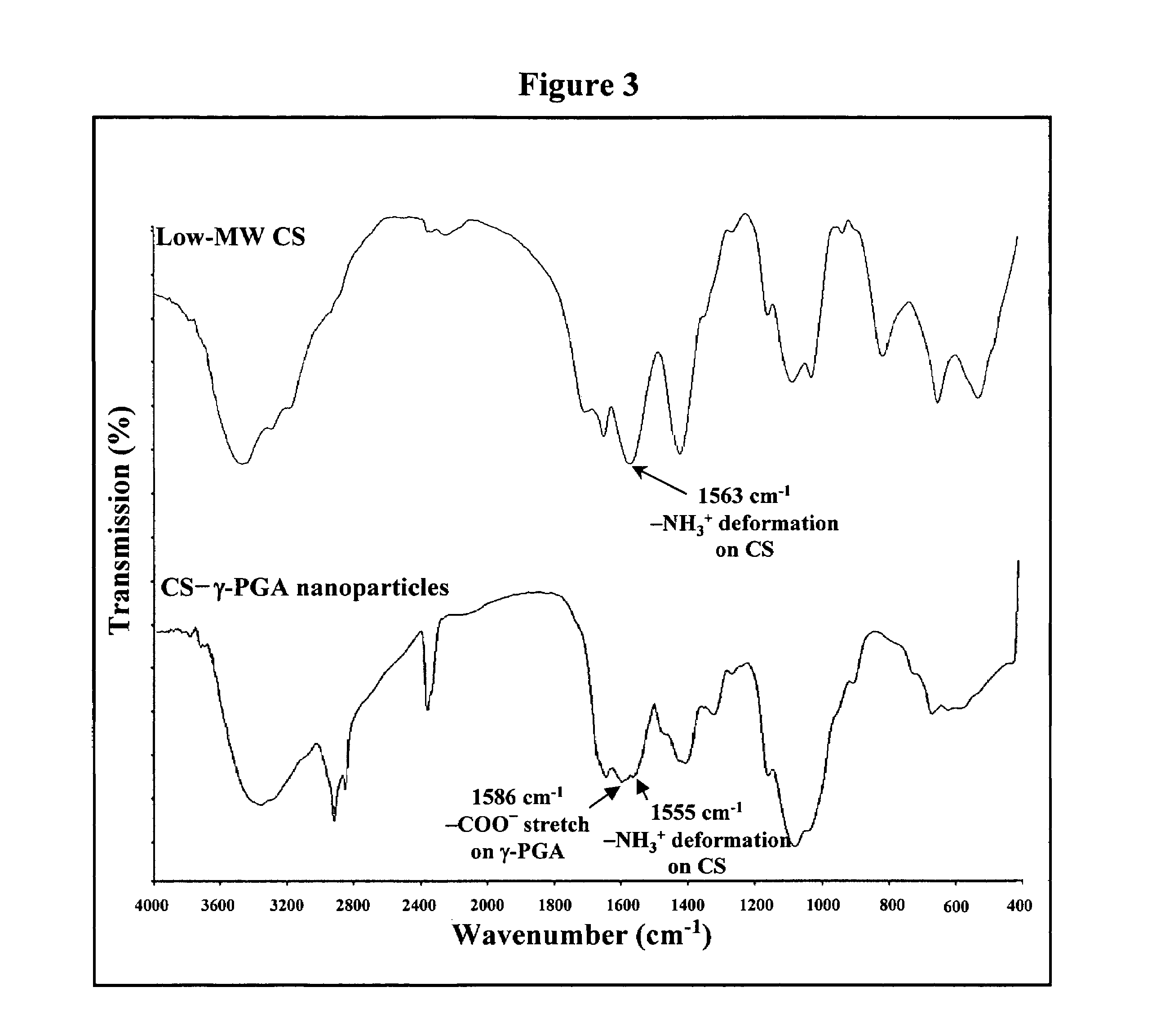
Nanoparticles for paracellular drug delivery
InactiveUS7265090B2Reduce resistanceEasy to transportAntibacterial agentsBiocideNanoparticleMedicine
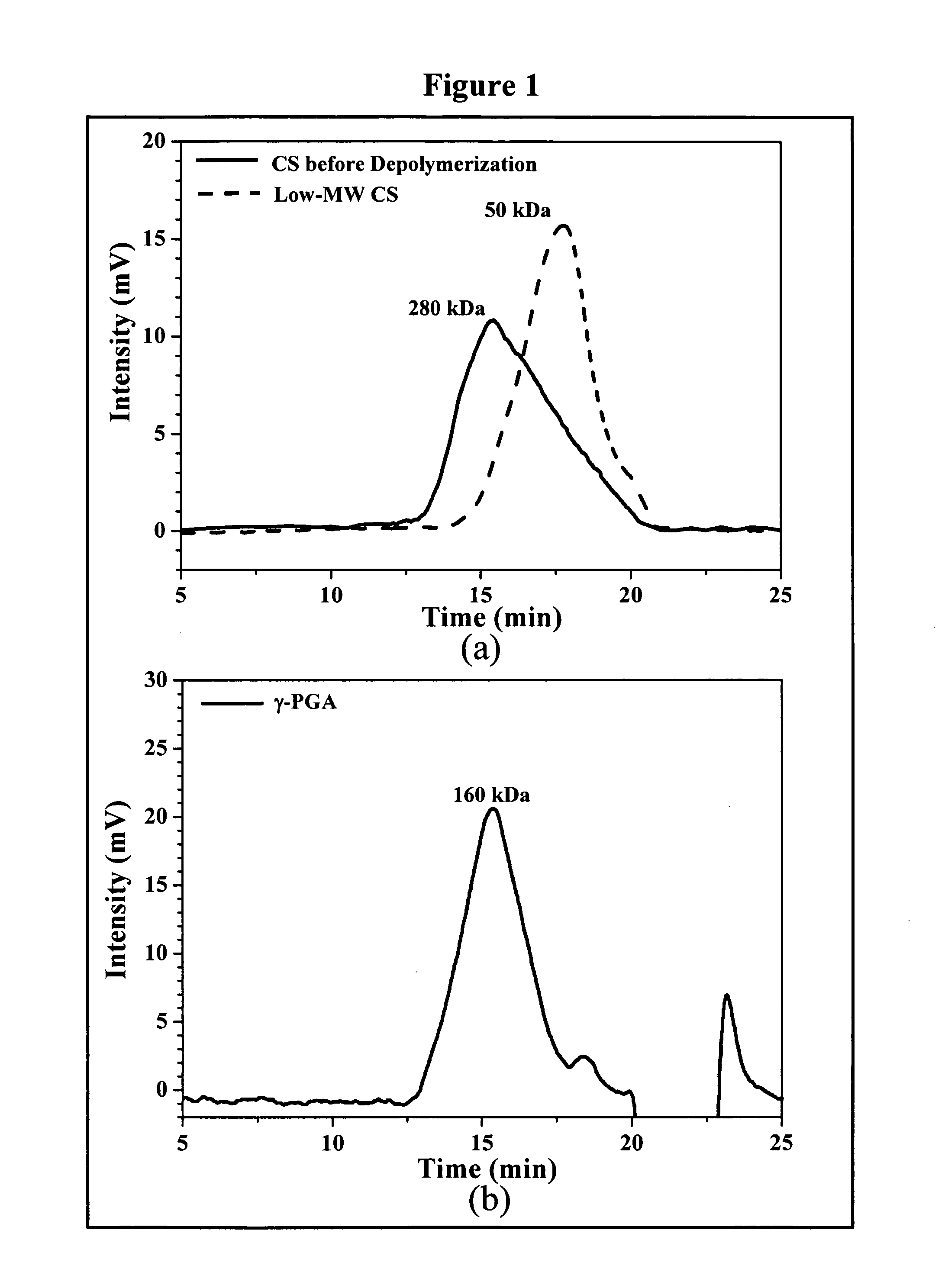
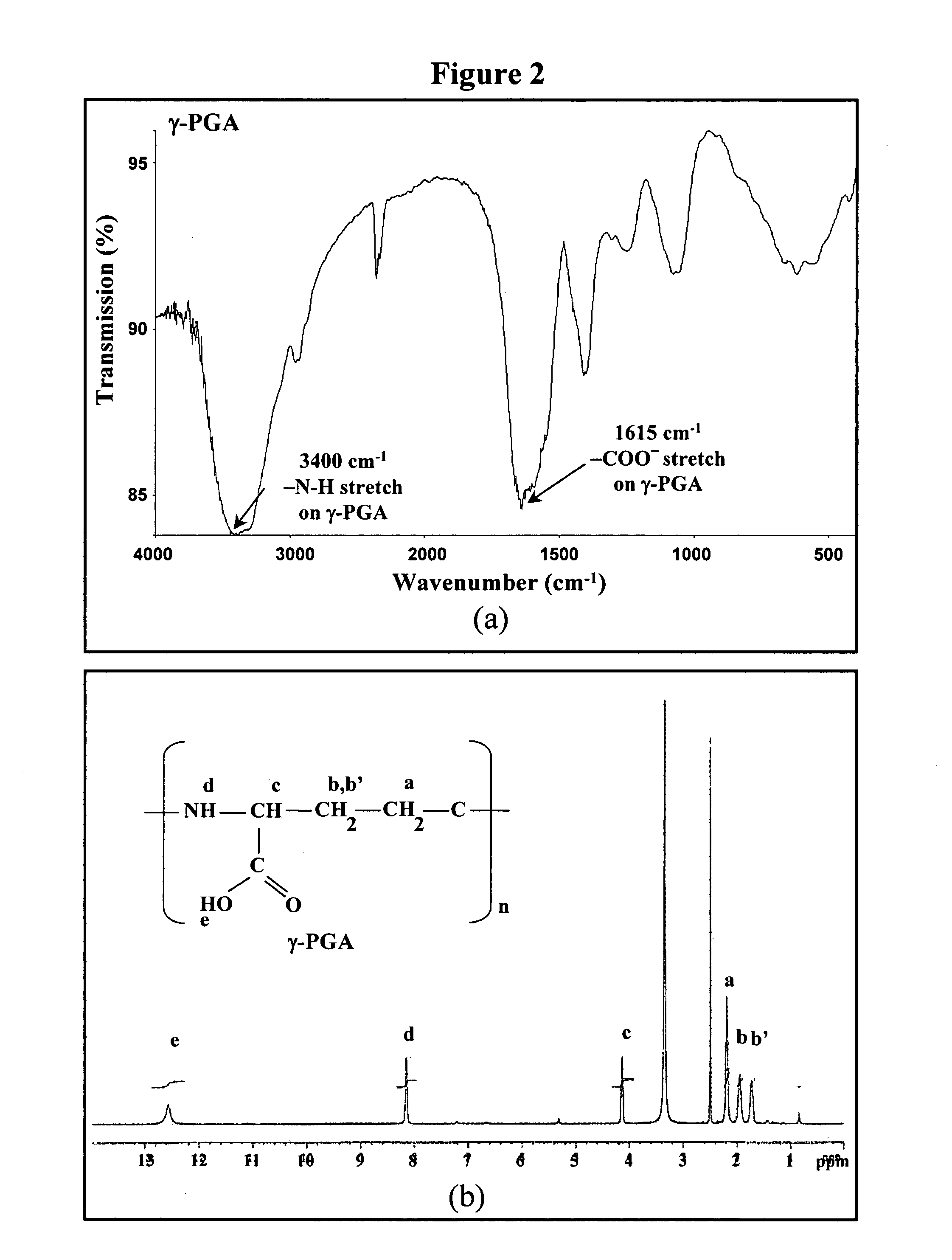
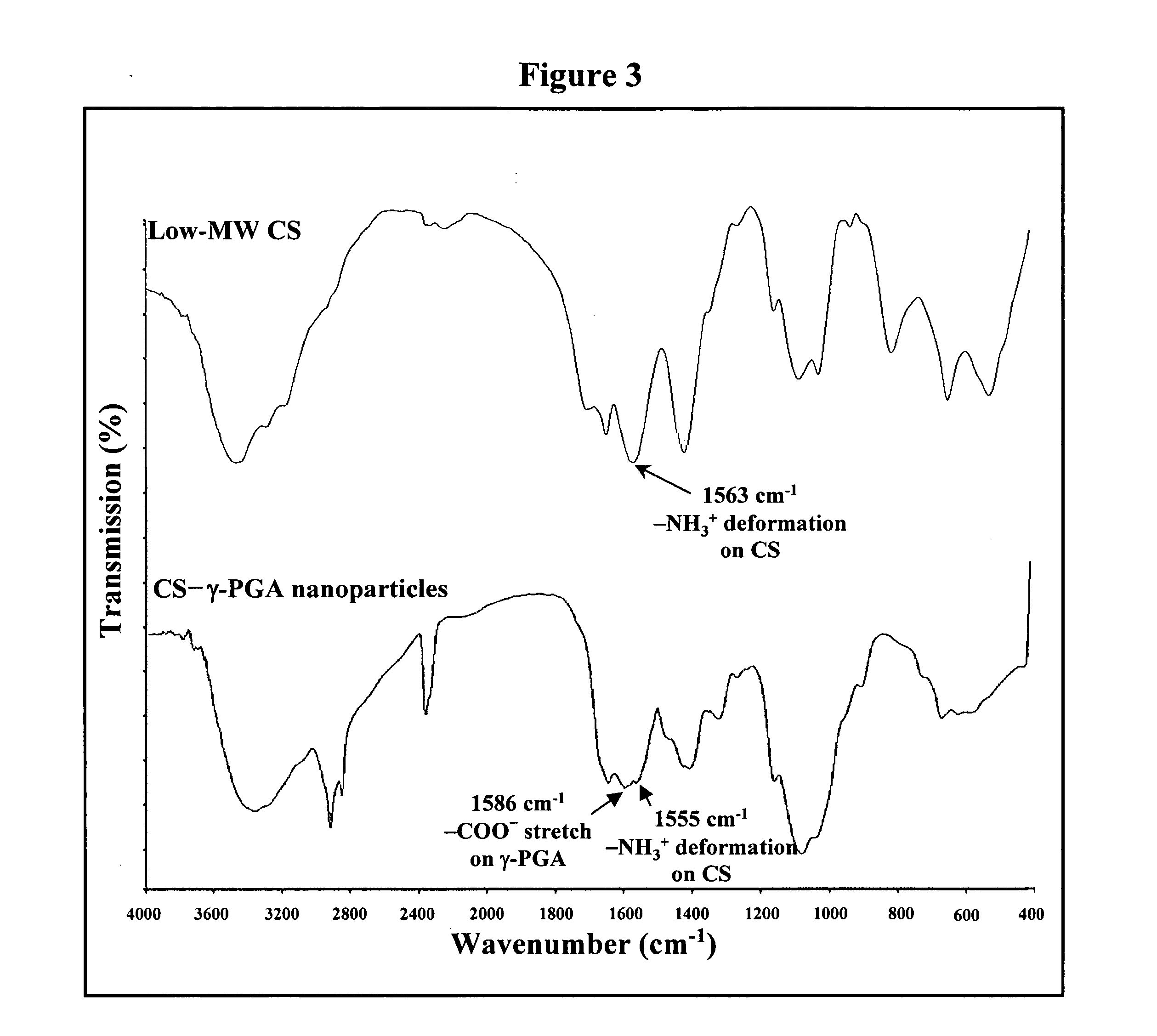
The invention discloses the nanoparticles composed of chitosan / poly-γ-glutamic acid characterized with a positive surface charge and their enhanced permeability through Caco-2 cells for paracellular drug delivery.
Owner:NANOMEGA MEDICAL CORP
Nanoparticles for paracellular drug delivery
InactiveUS20060073210A1Enhancing intestinal paracellular transportReduce resistanceAntibacterial agentsHeavy metal active ingredientsNanoparticleSurface charges
The invention discloses the nanoparticles composed of chitosan / poly-γ-glutamic acid characterized with a positive surface charge and their enhanced permeability through Caco-2 cells for paracellular drug delivery.
Owner:NANOMEGA MEDICAL CORP
Lactobacillus plantarum with abilities of reducing cholesterol and promoting intestinal short chain fatty acid generation ability and application of lactobacillus plantarum
ActiveCN108728382AImprove adhesionPromote growthBacteriaMicroorganism based processesMicroorganismMicrobiology
The invention relates to the technical field of microorganisms, and particularly discloses lactobacillus plantarum N-1 with abilities of reducing cholesterol and promoting intestinal short chain fattyacid generation ability and application of the lactobacillus plantarum. The lactobacillus plantarum N-1 was deposited in the China General Microbiological Culture Collection Center UNDER the accession number of CGMCC NO. 15463 with a deposit date of 20 Mar. 2018. The strain is separated from a traditional yak milk cheese product of Kalong Village, Mula Township, Daocheng County, Garze Tibetan Autonomous Prefecture, Sichuan Province, grows well on an MRS agar medium, has certain tolerance capacity on acid and bile salt, has high adhesion ability to human colon cancer cells Caco-2, has high cholesterol reducing ability, can remarkably improve generation of intestinal short chain fatty acids, is applied to the field of functional food, not only has actual production value, but also has quiteimportant significance on health of human bodies.
Owner:SICHUAN UNIV +1
Effective fractions of cicer arietinum linn bean sprout and preparation method and application thereof
ActiveCN102068486AImprove securityReduce processing costsAntineoplastic agentsFood preparationAdenocarcinoma colonApoptosis
The invention relates to effective fractions of cicer arietinum linn bean sprouts and a preparation method and application thereof. The effective fractions of the cicer arietinum linn bean sprouts, namely total saponin and total isoflavone are obtained by a plant chemical extraction separation method and a macroporous resin enrichment process. A bioactivity screening result indicates that the effective fractions of the cicer arietinum linn bean sprouts, namely the total saponin and the total isoflavone can suppress the growth of human adenocarcinoma colon strain Caco-2 cells by inducing cell apoptosis and are used for effectively inducing human adenocarcinoma colon cell apoptosis. A new thought for the research and development of the application of the effective fractions to the preparation of a medicament or a health-care product for treating colon cancer is provided.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
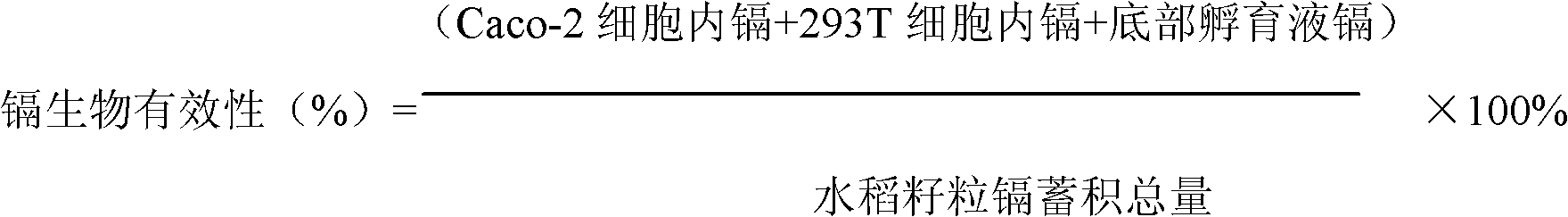
Method for building model for collectively evaluating bioavailability and toxicity of cadmium accumulation in food for human body
InactiveCN102680654AAccurate evaluationThe result is accurateMaterial analysis by electric/magnetic meansTesting foodCell layerChronic toxicity testing
The invention relates to a method for building a model for collectively evaluating bioavailability and toxicity of cadmium accumulation in food for a human body, and the method comprises the following steps of (1) food sample digestion and determination liquid acquiring: a, grinding a food sample after the food sample is collected, and sequentially digesting the food sample by sequentially adopting gastric juice and intestinal juice; and b, centrifuging the digested mixed liquid, collecting supernate to be sterilized through high pressure; (2) independently culturing Caco-2 cells and 293T cells; (3) combined culture of Caco-2 cells and 293T cells: moving an insertion groove with the Caco-2 cells into a hexagonal-porous plate with the 293T cells to be continuously cultured for 24h; (4) evaluation of bioavailability and toxicity of cadmium: placing the food sample determination liquid on a Caco-2 cell layer of a combined culture model, simultaneously adding iso-osmotic incubation liquid on a 293T cell layer, and continuing the culture for 24h; in the chronic toxicity test, maximally collectively culturing the sample for 10d; and (5) detecting indexes. The method has characteristics of simpleness in operation, easiness in controlling test conditions, small pollution, economical efficiency, accurate result and the like, and also has the advantages for collectively evaluating the bioavailability and toxicity. The method is suitable for evaluating the safety of the cadmium accumulation in grains, vegetables and animal food.
Owner:HENAN UNIV OF SCI & TECH
Targeting metabolic enzymes in human cancer
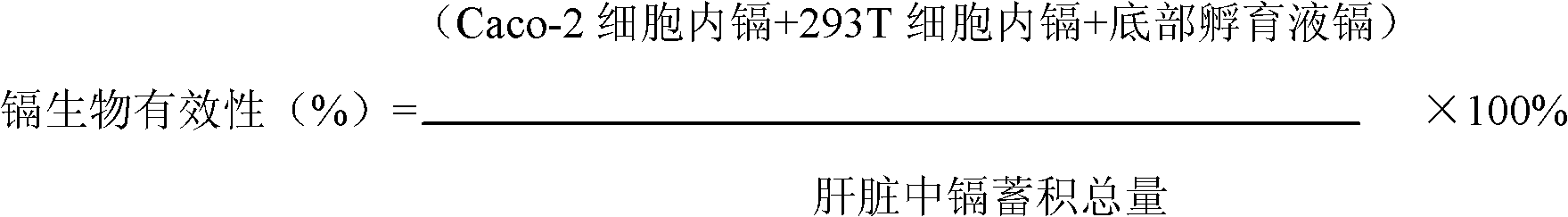
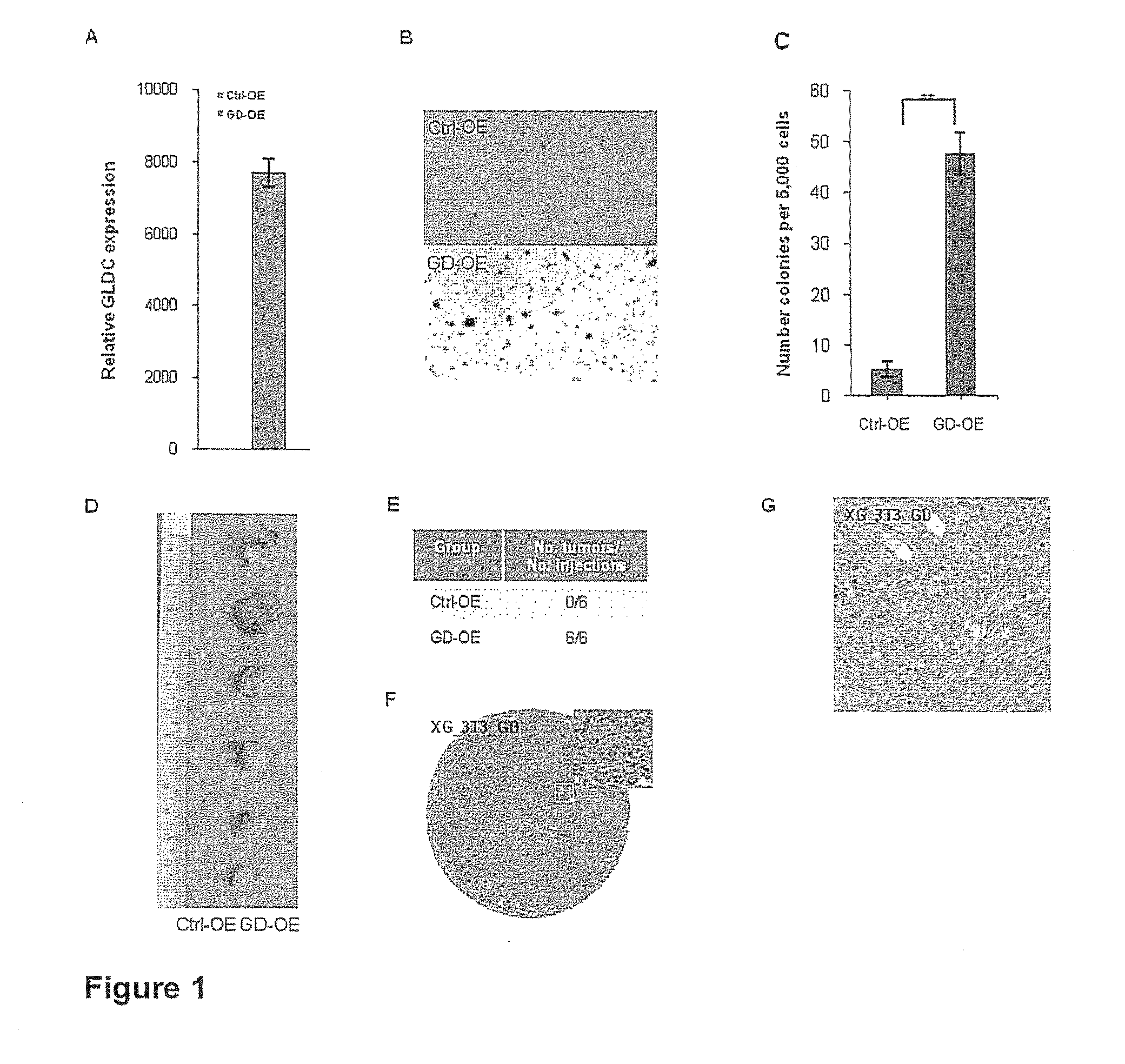
Targeting metabolic enzymes in human cancer Abstract Lung cancer is a devastating disease and a major therapeutic burden with poor prognosis. The functional heterogeneity of lung cancer (different tumor formation ability in bulk of tumor) is highly related with clinical chemoresistance and relapse. Here we find that, glycine dehydrogenase (GLDC), one of the metabolic enzyme involved in glycine metabolism, is overexpressed in various subtypes of human lung cancer and possibly several other types of cancers. GLDC was found to be highly expressed in tumor-initiating subpopulation of human lung cancer cells compared with non-tumorigenic subpopulation. By array studies we showed that normal lung cells express low levels of GLDC compared to xenograft and primary tumor. Functional studies showed that RNAi inhibition of GLDC inhibits significantly the clonal growth of tumor-initiating cells in vitro and tumor formation in immunodeficient mice. Overexpression of GLDC in non-tumorigenic subpopulation convert the cells to become tumorigenic. Furthermore, over-expression of GLDC in NIH / 3T3 cells and human primary lung fibroblasts can transform these cells, displaying anchorage-independent growth in soft agar and tumor-forming in mice. Not only is GLDC is expressed human lung cancer, it is also up-regulated in other types of cancer, such as colon cancer. RNAi knockdown of GLDC in colon cancer cell line, CACO-2 cells, can also inhibit the tumor formation in mice. Thus GLDC maybe a new metabolic target for treatment of lung cancer, and other cancers.
Owner:AGENCY FOR SCI TECH & RES
Two series major vault protein of glucagon-like peptide-2 of people and preparation method thereof
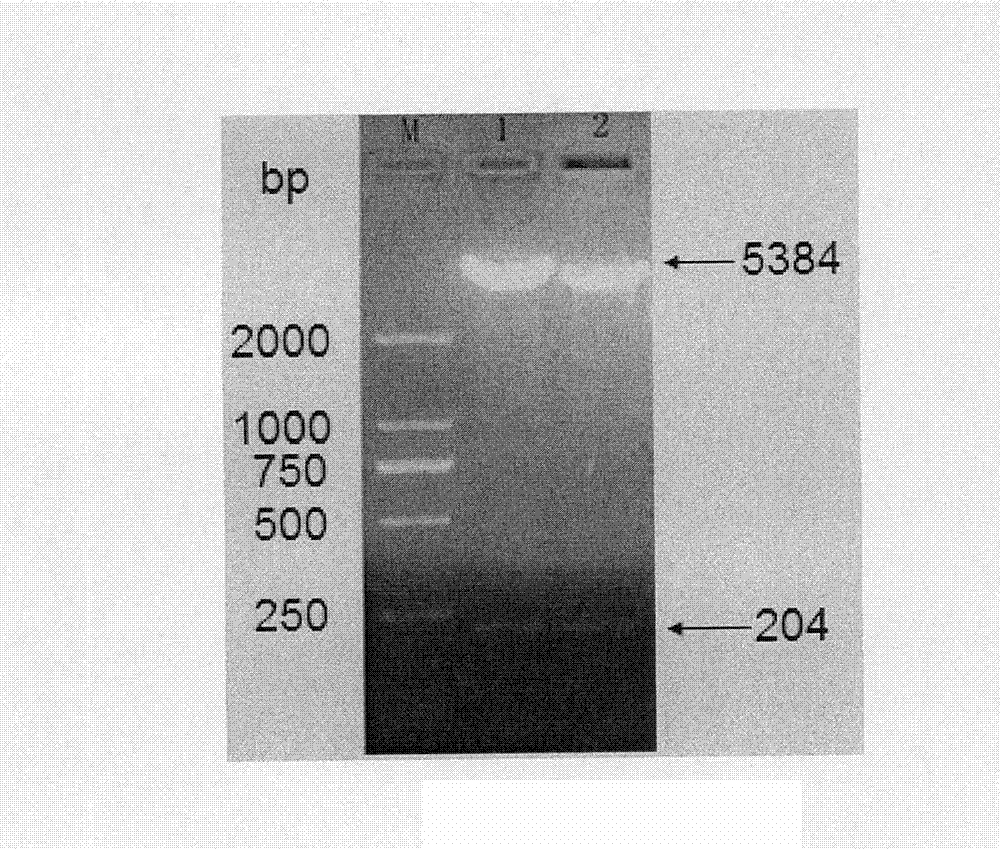
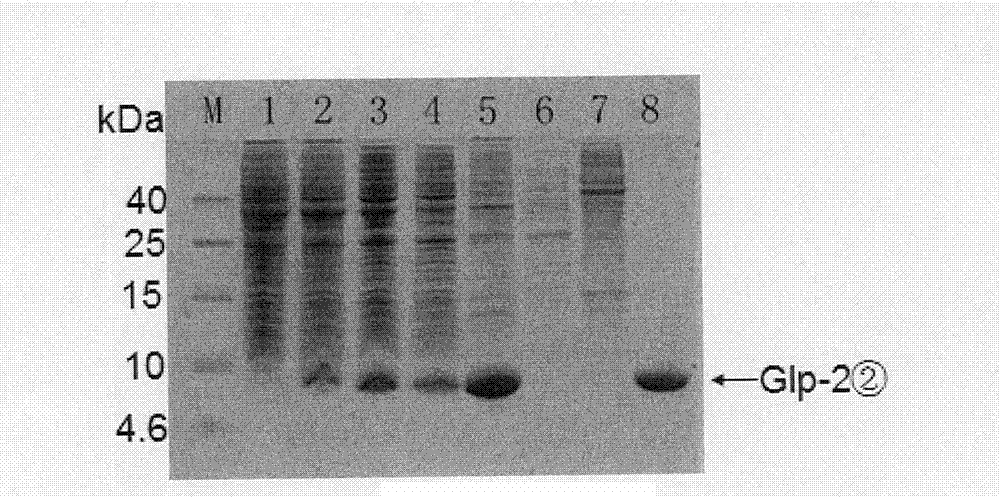
The invention belongs to the field of medical biotechnology, and relates to two series major vault protein of glucagon-like peptide-2 (GLP-2) of people and a preparation method of the two series major vault protein. A second place of an amino acid fragment of the glucagon-like peptide-2 of people is mutated, two segments are connected in series through connecting peptide, a dimmer structure of the GLP-2 is simulated, encoding gene of the two series major vault protein of the GLP-2 is obtained by a synthetic method, the encoding gene is cloned to a pET22b(+) pronucleus so as to express a vector, Escherichia coli BL21 is transformed, the encoding gene is expressed in the Escherichia coli efficiently, the purified reconstructed purpose protein is obtained through salt fractionation of ammonium sulfate in fractional and chromatography of anion exchange columns. The reconstructed purpose protein can stimulate a Caco-2 cell, multiplication of the Caco-2 cell can be promoted notably, the two series major vault protein of GLP-2 of people has natural biological activities, and therefore a foundation is established for further study and extensive use of the GLP-2 of people.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Formulations for enhanced bioavailability of orally administered polar agents
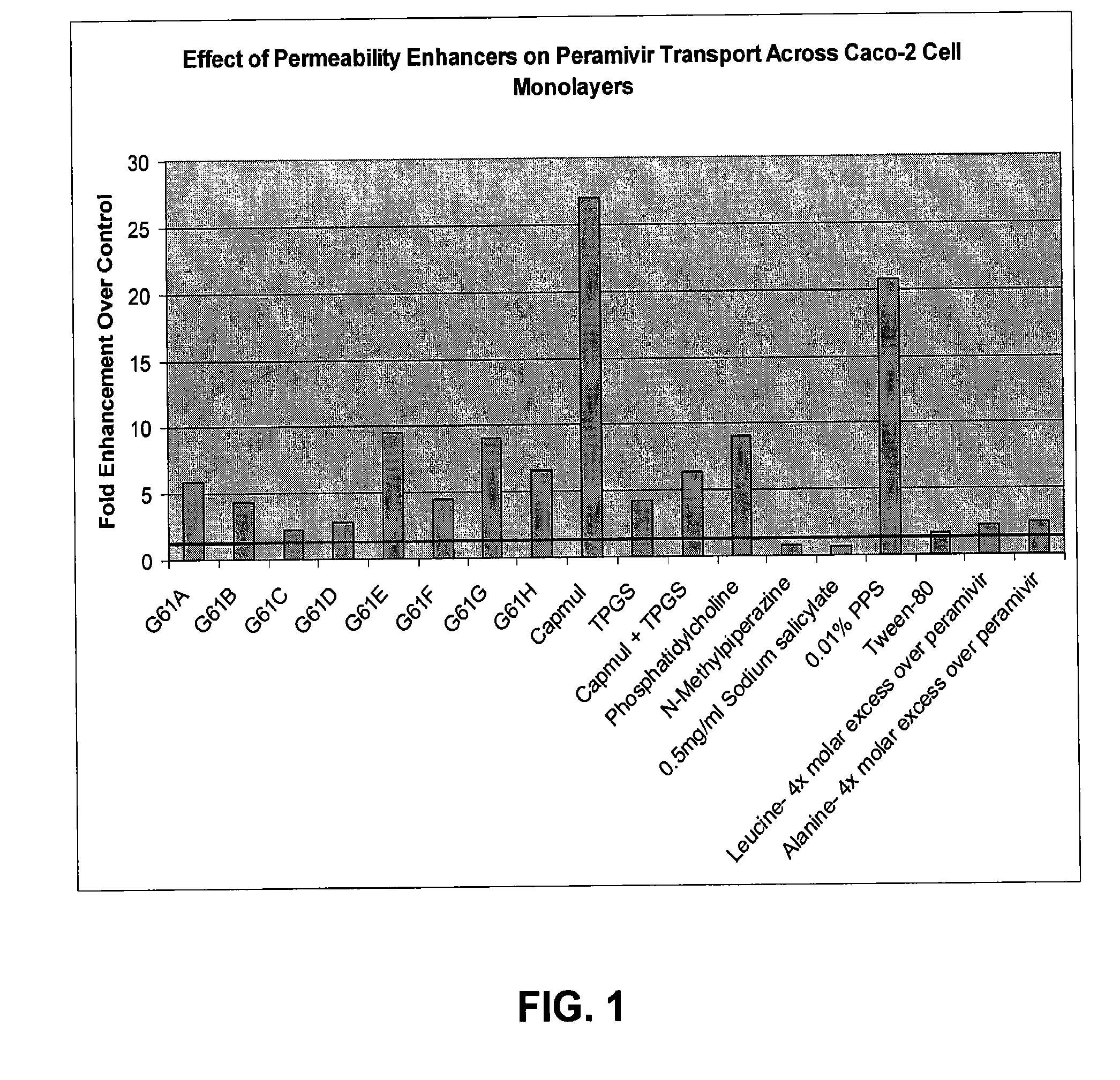
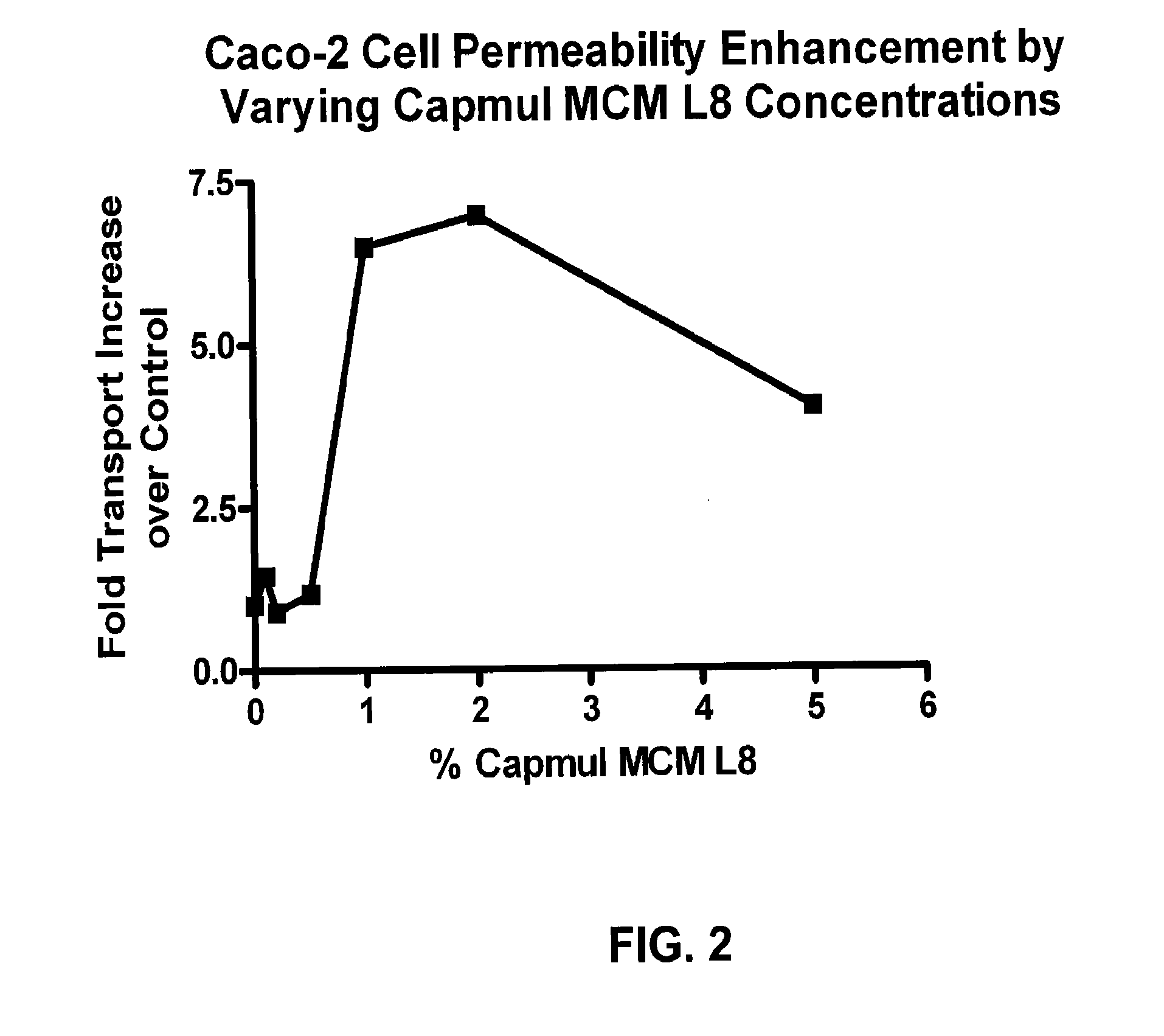
A composition is described having improved oral permeability of polar agents such as neuraminidase inhibitors. The composition includes one or more polar agents and one or more permeability enhancers such that the composition increases the amount of the polar agent capable of being transported across a Caco-2 cell membrane by at least 150% relative to the amount capable of being transported across the Caco-2 Cell membrane in the absence of the permeability enhancer. Oral dosage forms including the composition, and methods of treating or preventing influenza infection are also provided.
Owner:ALA WAI PHARMA
Protein delivery system of specific targeting macrophages
InactiveCN105983100ADoes not affect the identification mechanismReduce releasePeptide/protein ingredientsPharmaceutical non-active ingredientsAntigenDisease
The invention discloses a yeast hollow beta-glucan shell wrapped GMP-BSA protein targeting macrophage delivery system. The system allows BSA to be coated in a yeast shell through using a compact layer formed through the electrostatic adsorption effect of chitosan (CS), tripolyphosphate (TPP), sodium alginate and BSA. GMP-BSA particles prepared in the invention have very good protein release behavior in in-vitro experiments, and can avoid protein loss. The particles highly specifically target the macrophages, such as Raw 264.7 cells, primary BMDM cells (primary bone marrow macrophages) and peritoneal macrophages (PEMs), and are not devoured by NIH3T3, AD293, HeLa, Caco-2 and other non-macrophage tumor cells, and neutral granulocytes in blood. The macrophages play a great role in the pathogenesis of various diseases, and are very important potential target spots. The macrophages are key antigen transfer cells, and are very important in vaccine design. The system can highly selectively deliver various proteins to the macrophages in a targeting manner, and also has very wide application prospect in the field of targeting drug administration and the field of macrophage vaccine design.
Owner:NANKAI UNIV
Determination method for absorption and transportation amount of six components in rhizoma bletillae in Caco-2 cell model
ActiveCN105954411AEvaluation of in vivo absorption propertiesComponent separationInternal standardIn vivo absorption
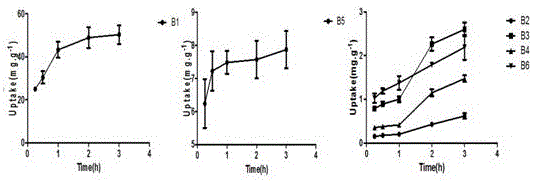
The invention discloses a determination method for the absorption and transportation amount of six components in rhizoma bletillae in a Caco-2 cell model. The method comprises the steps of: preparing a rhizoma bletillae extract solution, a standard solution serving as a reference substance and an internal standard solution; establishing a human-derived colon adenocarcinoma cell line Caco-2 cell model; preparing a cell suspension through a Caco-2 cell model; determining the content of the six components by UPLC-MS / MS; determining the total protein content according to a Coomassie brilliant blue dye liquor protein determination kit method, and calculating the cell uptake X=the total protein of a to-be-determined substance. The invention adopts UPLC-MS / MS to establish the analysis method for the 6 components in a rhizoma bletillae extract, determines the influence of the rhizoma bletillae extract to absorption and uptake of Caco-2 cells under the conditions of time, concentration, temperature, pH and P-gh inhibitors, preliminarily evaluates the in vivo absorption characteristics of the rhizoma bletillae extract, and provides scientific basis for the research and development of the oral preparation of the rhizoma bletillae extract.
Owner:GUIZHOU MEDICAL UNIV
Method for quantitatively evaluating antioxidant activity of antioxidant based on Caco-2 cell model
InactiveCN103760144AEffective analysis and evaluation toolsFluorescence/phosphorescenceFluorescenceAntioxidant
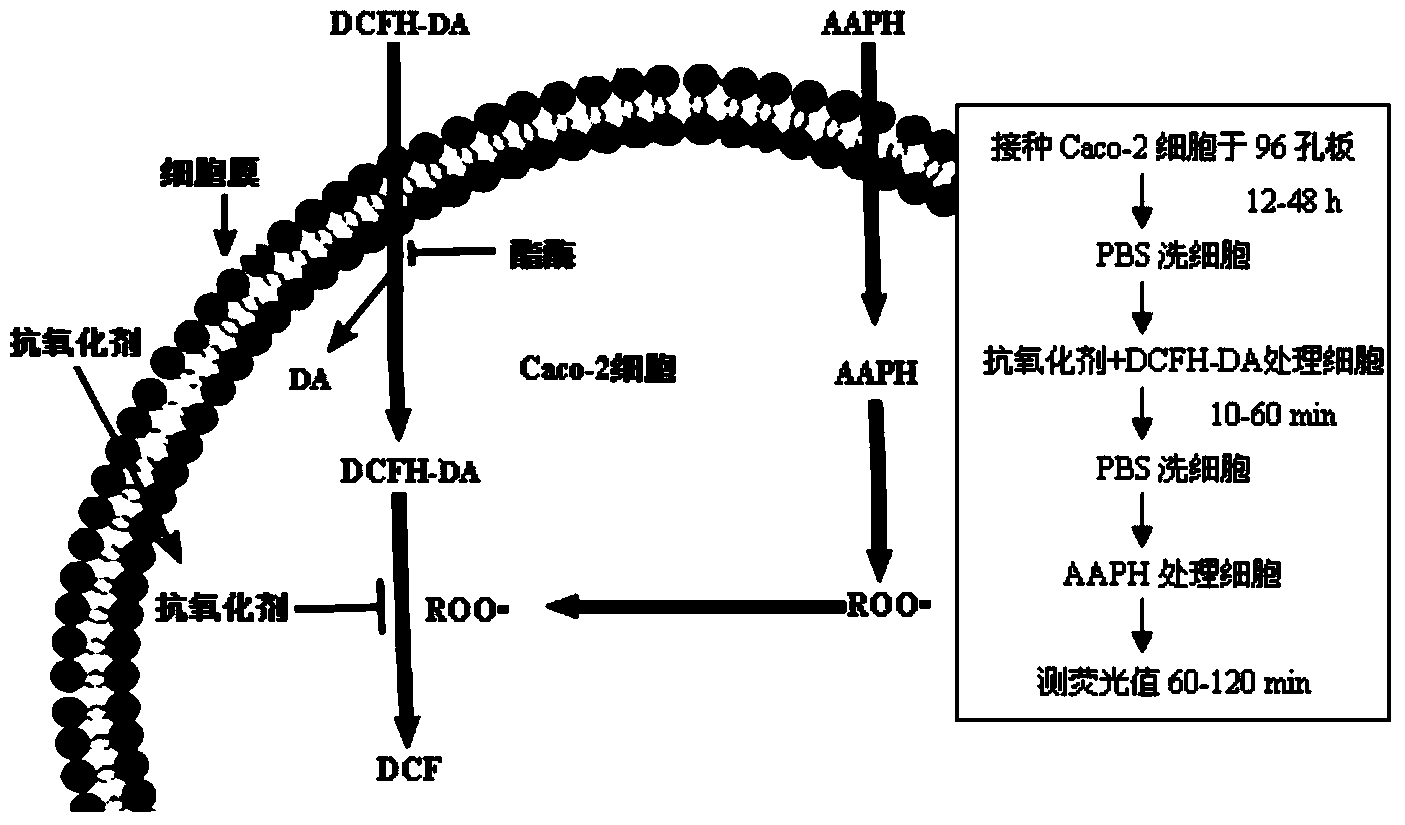
The invention discloses a method for quantitatively evaluating the antioxidant activity of an antioxidant based on a Caco-2 cell model, relating to an antioxidant. The method for cytologically and quantitatively evaluating the antioxidant activity of an antioxidant based on a Caco-2 single-layer cell model is established by using the Caco-2 cell model and optimizing the inoculation concentration and culture time of cells as well as the incubation conditions of a fluorescent probe (2',7'-dichlorodihydrofluorescein diacetate, DCFH-DA for short), a free radical initiator (2,2'-azobis(2-methylpropionamidine)dihydrochloride, AAPH for short), a pure antioxidant and Caco-2 cells. The method has the advantages of high biological relevance, easiness and high speed, and an effective analysis and evaluation tool is provided for the research of biological antioxidants.
Owner:SHENZHEN POLYTECHNIC
Construction method for human intestinal tract epithelial cell model
ActiveCN110628702AAccurate responseClear responseGastrointestinal cellsEpidermal cells/skin cellsHuman body3D cell culture
The invention discloses a construction method for a human intestinal tract epithelial cell model, and relates to the technical field of cell model construction. The human intestinal tract epithelial cell model is characterized in that a Caco-2 cell is taken as a model cell, and a DMEM (dulbecco's modified eagle medium) containing 20%FBS (fetal calf serum) is used for carrying out 3D culture on theCaco-2 cell in a micro-fluidic chip so as to construct the in vitro model of the human intestinal tract epithelial cell. On one hand, a human cell is adopted for culture, the problem that an experiment result is in accurate since an animal cell is adopted for culture is avoided, and on the other hand, the 3D cell culture enables intestinal tract cells to own a mechanical microenvironment which ismore similar to the human physiology, so that intestinal tract cells can be accelerated to differentiate. Therefore, the human intestinal tract epithelial cell model constructed by the construction method disclosed by the invention can more clearly and accurately reflect the characteristics of small intestine epithelial barriers.
Owner:WUHAN CHOPPER BIOLOGY
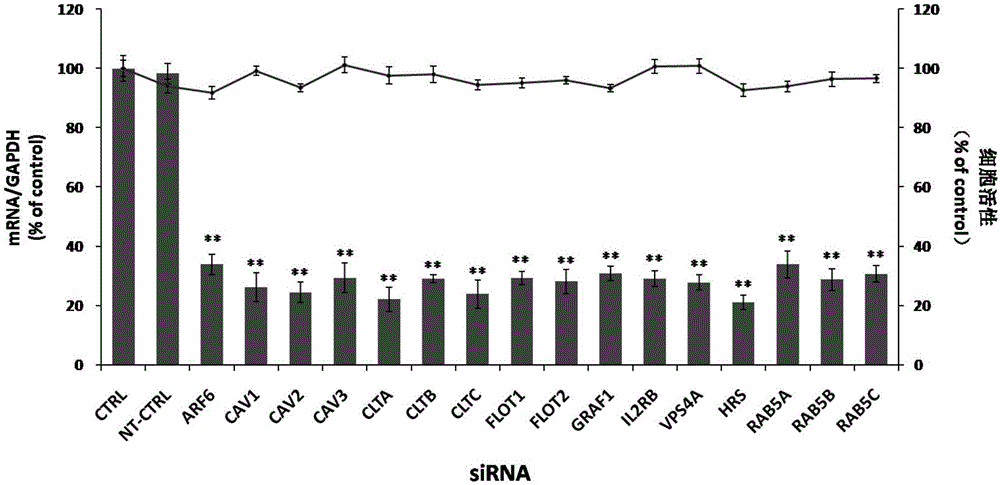
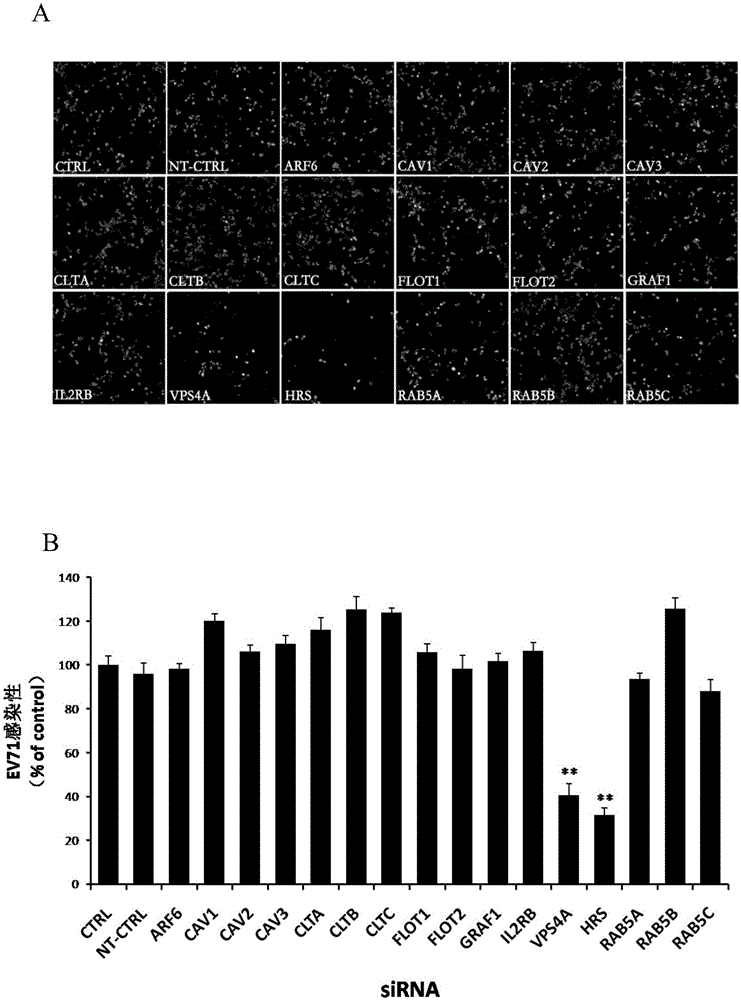
Applications of hepatocyte growth factor-regulated tyropsine kinasesubstrate in preparation of medicines preventing enterovirus 71-type infection
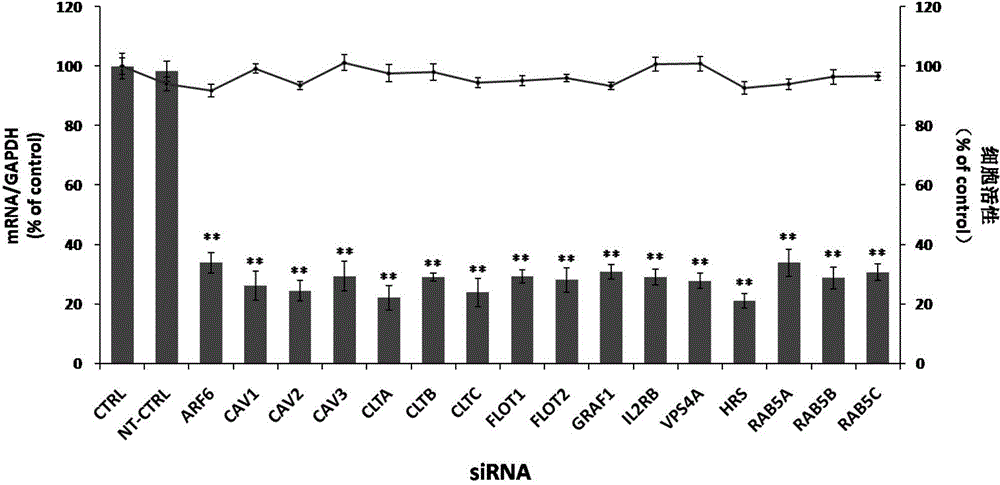
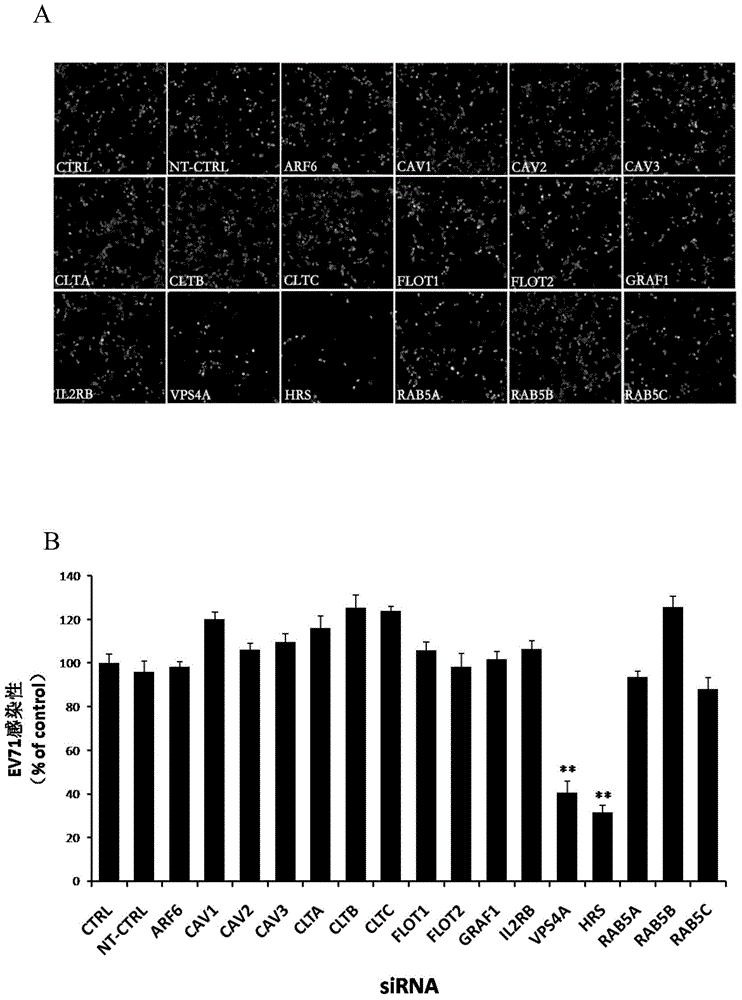
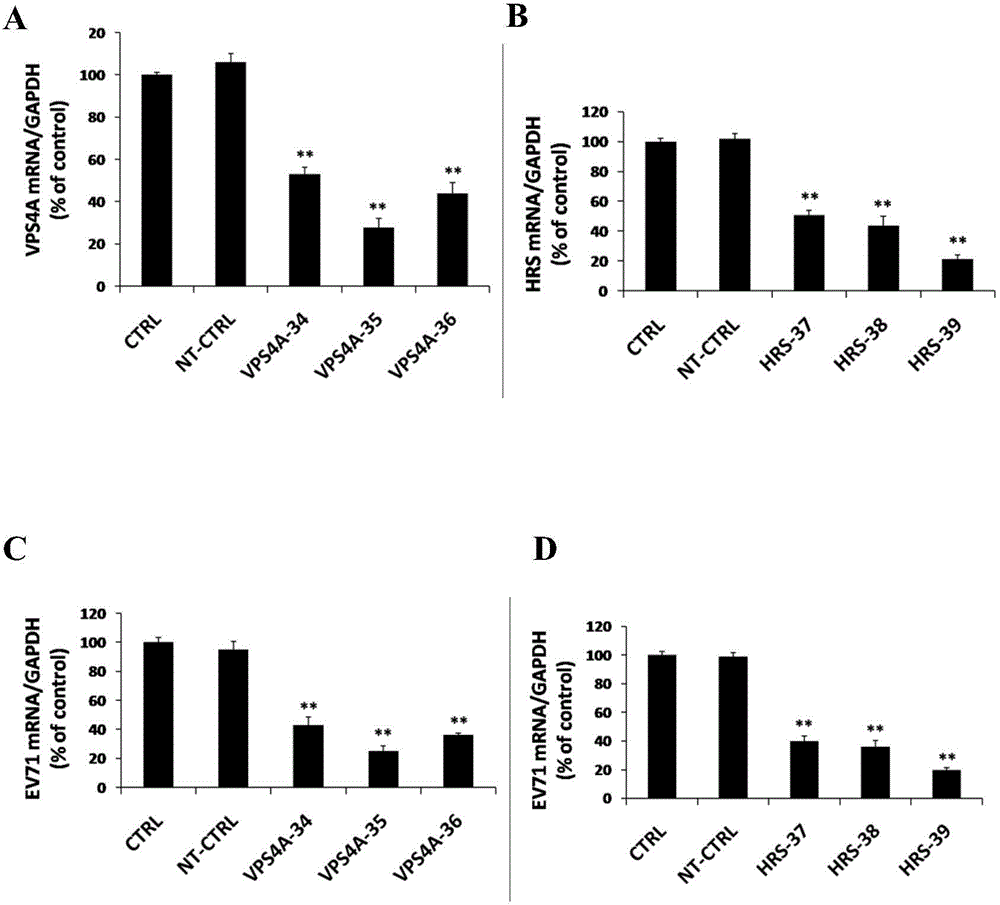
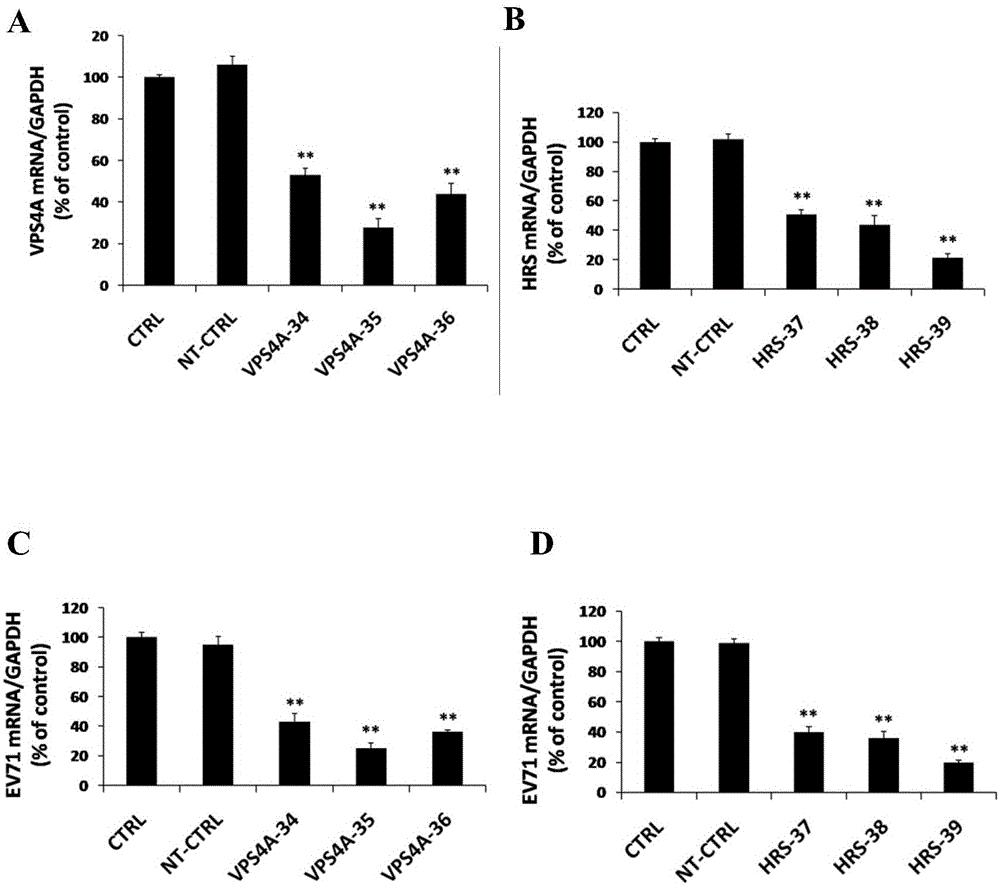
The invention relates to the biomedical technology field, and provides a new target spot for resisting enterovirus 71-type infection and applications. Human colon cancer cells (Caco-2) are employed as target cells, the RNA interference technology is employed to reduce expression of the target cell host proteins, a host factor capable of inhibiting EV71 infection of human colon cancer cells (Caco-2) effectively is sought and the purpose of cutting off EV71 infection from the source (intestinal tract) effectively is achieved. Experiments show that hepatocyte growth factor-regulated tyropsine kinasesubstrate (HRS) plays an important role in inhibiting EV71 infection of Caco-2, the HRS expression is reduced, and EV71 infection can be inhibited obviously. Applications of HRS in preparation of medicines preventing or treating enterovirus 71-type infection are provided.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Exopolysaccharide-producing pediococcus pentosaceus and application thereof
ActiveCN106222103AStrong adhesionImprove antioxidant capacityMilk preparationBacteriaPediococcus speciesStressed state
The invention discloses exopolysaccharide-producing pediococcus pentosaceus and application thereof, and belongs to the field of microbes. The pediococcus pentosaceus provided by the invention has been preserved in CGMCC (China General Microbiological Culture Collection Center) from December 11, 2015, and a preservation number is CGMCC No:11857. A strain has the advantages that high yield of the exopolysaccharide can be realized; the exopolysaccharide yield is 153.60 mg / L. The strain has high resistant capability on NaCl, cholate and gastrointestinal fluid, and has high adhesion capability on Caco-2 cells; in addition, the oxidation resistance capability on HepG2 cells in an oxidation stress state is improved; the pediococcus pentosaceus can be used as probiotics or oxidation resistance products to be used in the fields of food, medicine, cosmetics and the like.
Owner:SHAANXI SCI TECH UNIV +1
Food allergen dynamic digestion model and in-vitro simulation evaluation method
InactiveCN110531059AReduce experimental errorReduce space station ratioBiological testingHuman bodyColonic adenocarcinoma
The invention discloses a food allergen dynamic digestion model and an in-vitro simulation evaluation method. The method mainly comprises the steps of establishing an allergen dynamic digestion modeland an in-vitro simulation evaluation method, simulating a biochemical reaction condition of the stomach and duodenum of a human body through a digestive system simulation device, providing a method for constructing an allergen in-vitro simulated dynamic digestion experiment in the simulated evaluation method, constructing a Caco-2 (human cloned colonic adenocarcinoma cells) small intestine epithelial cell model to simulate small intestine absorption and transport effects, and constructing a KU812 (human peripheral blood basophilic leukemia cells) cell model to evaluate the ability of mast cell degranulation induced by food allergen digestion and transport products. The method of the invention is suitable for evaluating the digestion and sensitization of a purified food allergen or an allergen extracting solution containing a complex food matrix.
Owner:OCEAN UNIV OF CHINA
Method for measuring absorption and transporting quantities of effective ingredients in herba-erigerontis and red-peony-root extract in Caco-2 cell model
InactiveCN106153799APromote absorptionAbsorption increases and decreasesComponent separationReference sampleApigenin
The invention discloses a method for measuring the absorption and transporting quantities of effective ingredients in herba-erigerontis and red-peony-root extract in a Caco-2 cell model. the method includes the steps that herba-erigerontis and red-peony-root extract testing liquid is prepared; a series of standard solutions serving as reference samples are prepared from albiflorin std, gallic acid, caffeic acid, scutellarin, scutellarin and apigenin-7-o-glucronide; an internal standard solution is prepared from puerarin; then the human-source intestinal-gland cancer cell line Caco-2 cell model is built; the obtained testing liquid and the series of standard solutions are respectively added into the Caco-2 cell model, and after a cell lysis solution is added for cell lysis, a medicine-containing cell suspension is obtained; the absorption quantities of five active ingredients in the obtained medicine-containing cell suspension are measured through a UPLC-MS. According to the method, in the test, the Caco-2 cell model is combined with the LC-MS technology to research the absorption mechanism of the five active ingredients in the herba-erigerontis and red-peony-root extract, the characteristics of taking, transmembrane transporting and the like of the ingredients are studied, and the comprehensive absorption information of medicine molecules is provided in the cell level.
Owner:GUIZHOU MEDICAL UNIV
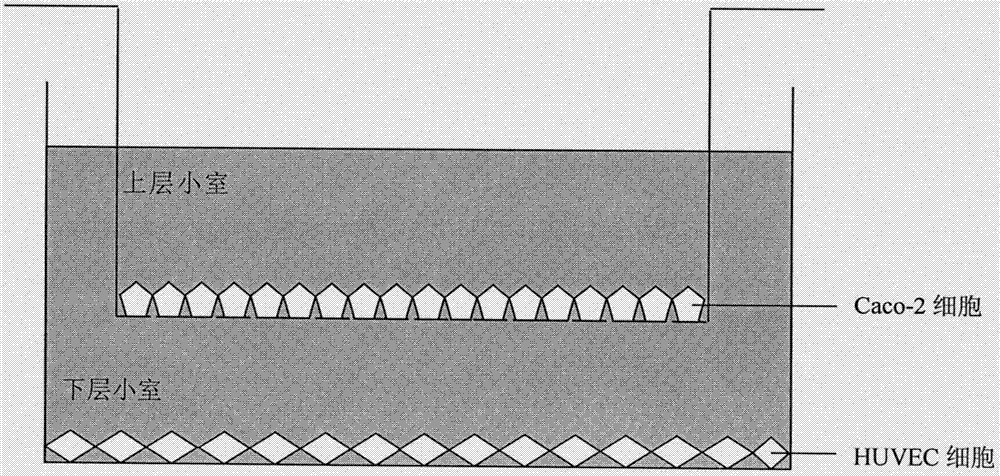
Method for constructing Caco-2/HUVEC cell co-culture system
InactiveCN107083364AComprehensive reflection of bioavailabilityTrue reflection of bioavailabilityCulture processArtificial cell constructsHUVEC CellsBioavailability
The invention discloses a method for constructing a Caco-2 and HUVEC cell co-culture system. The method comprises inoculating an upper small cell of a Transwell plate with 25 to 40 passages of Caco-2 cells in a logarithmic phase, carrying out culture until the cells are completely differentiated, inoculating a lower small cell of the Transwell plate with 2 to 6 passages of HUVEC cells in a logarithmic phase, carrying out culture until 80-90% of the lower small cell is filled with the cells, inserting the completely differentiated Caco-2 cell Transwell upper small cell into the cultured HUVEC cell Transwell lower small cell, fine adjusting a DMEM medium and carrying out non-contact co-culture at 37 DEG C in 5% of CO2. The Caco-2 / HUVEC co-culture system realizes simultaneous research of absorption, metabolism and physiological activity of drugs, simulates the pharmacological action of the drug in the circulatory system after absorption and metabolism in the intestinal tract and provides a cell model for showing the bioavailability of the drug.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Evaluation model for zinc biological effectiveness in foods and establishment method thereof
InactiveCN102676369ABioreactor/fermenter combinationsBiological substance pretreatmentsEvaluation resultHuman intestinal absorption
The invention relates to an evaluation model for zinc biological effectiveness in foods and an establishment method thereof. The model comprises a model structure, cell types and evaluation indexes, wherein the evaluation indexes are the zinc content in Caco-2 cells, cell transported zinc quantity and the zinc content in epithelial cells of internal organs. The model can accurately, efficiently and comprehensively simulate the zinc absorbing and utilizing process in a human body and provides a reasonable and correct method for evaluating the zinc biological effectiveness in the foods; the model structure is similar to a human intestinal absorption structure; the model can evaluate the absorption efficiency of zinc in the foods in an intestinal tract and also can evaluate the utilization efficiency of the zinc in the human body; and evaluation results are close to the actual zinc biological effectiveness.
Owner:HENAN UNIV OF SCI & TECH
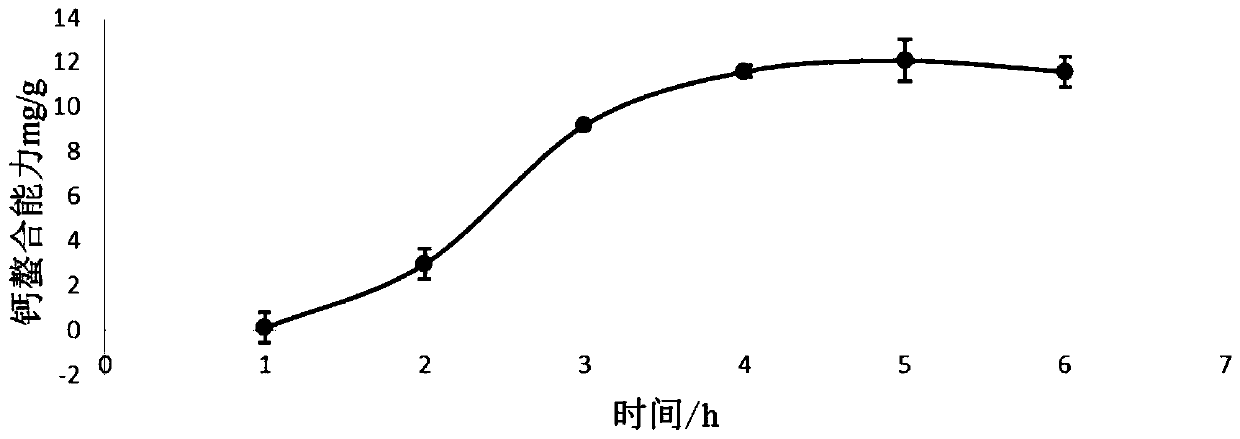
Calcium chelating peptide as well as preparation method and application thereof
ActiveCN110627896AImprove absorption and utilizationImprove bioavailabilityConnective tissue peptidesPeptide/protein ingredientsCalcium EDTAAmino acid
The invention relates to a calcium chelating peptide as well as a preparation method and an application thereof. An amino acid sequence of the calcium chelating peptide is FDHIVY. The calcium chelating peptide provided by the invention is safe and non-toxic, and has better physicochemical activity compared with a traditional calcium supplement, and the calcium chelating capability of the calcium chelating peptide reaches 35.73 mg / g; the collagen peptide can be supplemented while absorption and bioavailability of calcium in a human body are improved, the absorption and transport of calcium in Caco-2 small intestinal epithelial cells can be promoted, and the calcium chelating peptide can be used as a raw material for drug development and biological calcium supplementation agents. According to the preparation method provided by the invention, the calcium chelating peptide is successfully obtained, fish product resources can be fully utilized, the operation is simple, and a new way is provided for high-valued utilization of tilapia bones.
Owner:SOUTH CHINA AGRI UNIV
Bio-safety evaluation method of nano zinc oxide based on Caco-2 cells
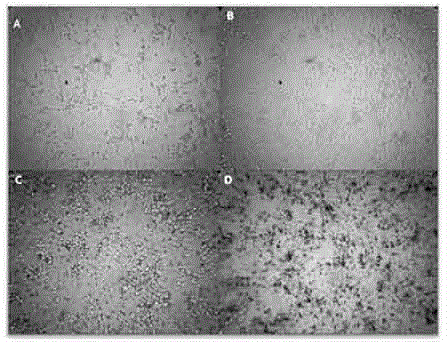
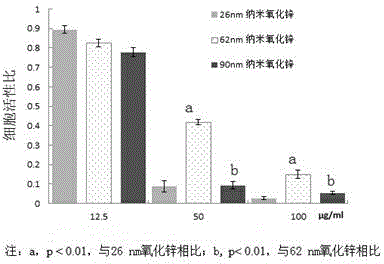
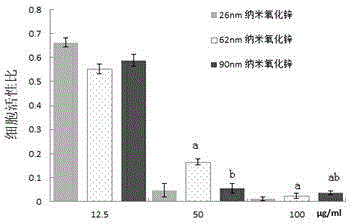
The invention provides a bio-safety evaluation method of nano zinc oxide based on Caco-2 cells. The method comprises the following steps: conventionally culturing cells, passaging the cells, freezing the cells, thawing the cells, contaminating the cells, performing morphological observation and detecting the cell activity. The method has certain theoretical meaning on discussion on intestinal toxicity of nano zinc oxide; a reference is provided for establishment of food nano zinc oxide limit standards, a reliable scientific basis is provided for further study on the safety problem of food nano zinc oxide, a reference is provided for safety evaluation of other nanomaterials, and theoretical support is provided for safety use of nano zinc oxide.
Owner:CHINA JILIANG UNIV
Establishing method for cell model used for researching drug absorption under plateau anaerobic condition
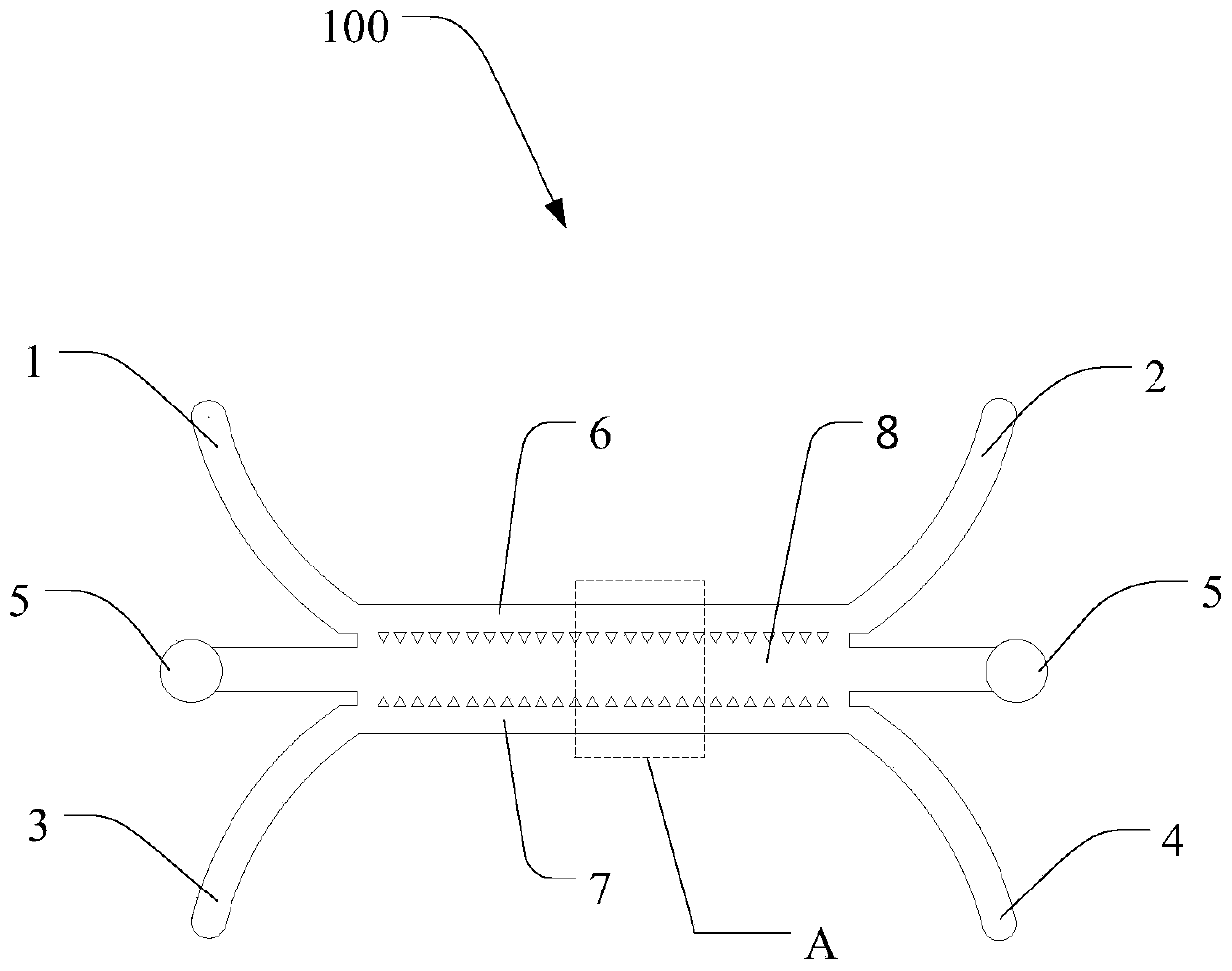
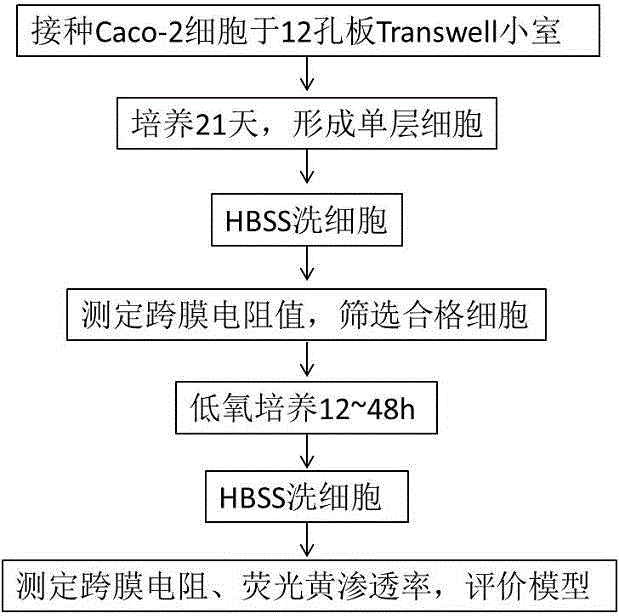
InactiveCN106701683AEasy to operateEnables high-throughput assaysTumor/cancer cellsOsmotic coefficientPlateau
The invention relates to an establishing method for a cell model used for researching drug absorption under a plateau anaerobic condition. The establishing method comprises the following steps: (1) culturing Caco-2 cells in a high-sugar DMEM culture solution; (2) constructing a Caco-2 cell single-layer model on a filter membrane of a 12-pore plate Transwell cell by the Caco-2 cells obtained in the step (1); (3) washing the Caco-2 cell single-layer model by HBSS; (4) measuring transepithelial electrical resistance of the Caco-2 cell single-layer model obtained in the step (3), and determining the Caco-2 cell single-layer model as a qualified Caco-2 cell single-layer model when the transepithelial electrical resistance is greater than 400 ohm; (5) performing anaerobic culture on the qualified Caco-2 cell single-layer model; (6) cleaning the Caco-2 cell single-layer model subjected to the anaerobic culture in the step (5); and (7) measuring the transepithelial electrical resistance and an fluorescein apparent permeability coefficient of the Caco-2 cell single-layer model obtained in the step (6), and completing model evaluation. The establishing method is simple to operate, is economic and scientific, can realize high-throughput measurement, administrates drugs for highland people in an individualized mode, and has relatively great promotion effect on reasonable drug administration.
Owner:王荣
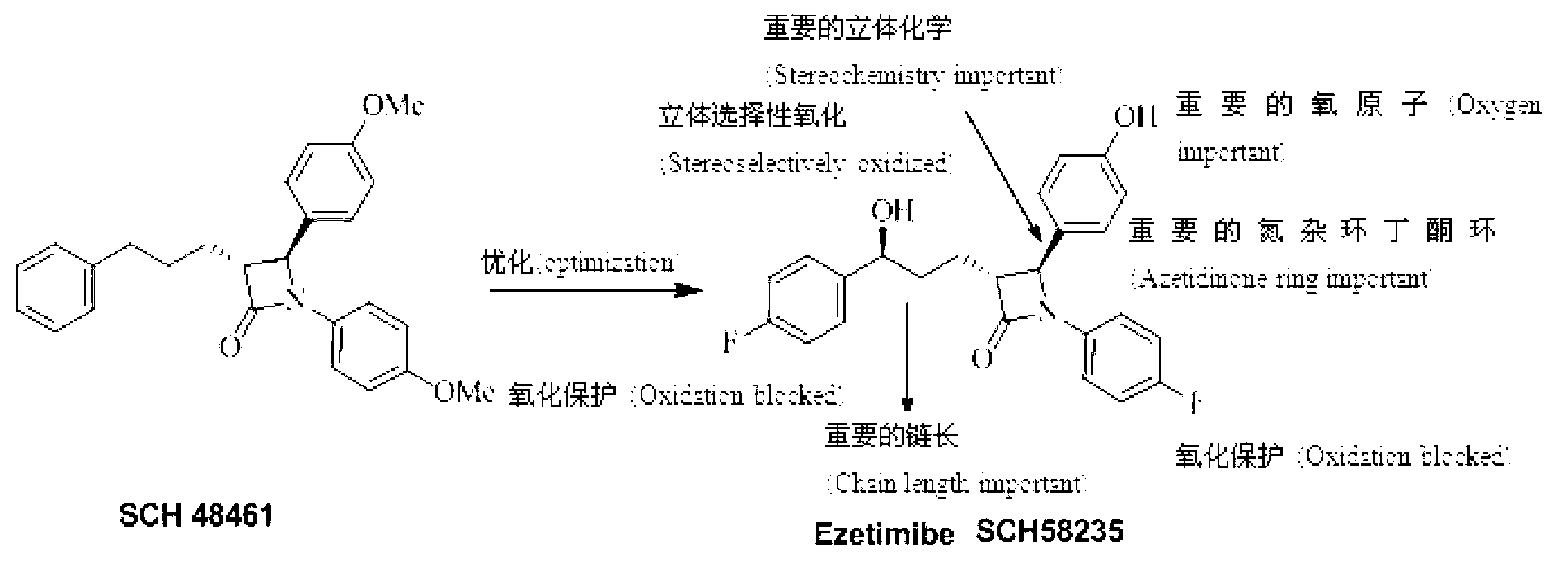
Ezetimibe analogue and preparation method thereof
InactiveCN103254107AAvoid absorptionLower cholesterol levelsOrganic chemistryMetabolism disorderHydrogen atomCholesterol absorption inhibitor
The invention relates to an ezetimibe analogue and a preparation method thereof. The structure general formula of the ezetimibe analogue is shown in a structural formula, wherein in the formula, R1, R2 and R3 are selected from fluorine atom, hydroxyl, hydrogen atom and methoxy independently. The ezetimibe analogue is an ideal inhibitor for sucking cholesterol, and the cholesterol inhibitor can obviously prevent Caco-2 cells from sucking the cholesterol, so that the ezetimibe analogue has the prospect of becoming the inhibitor for sucking the cholesterol.
Owner:DONGHUA UNIV +1
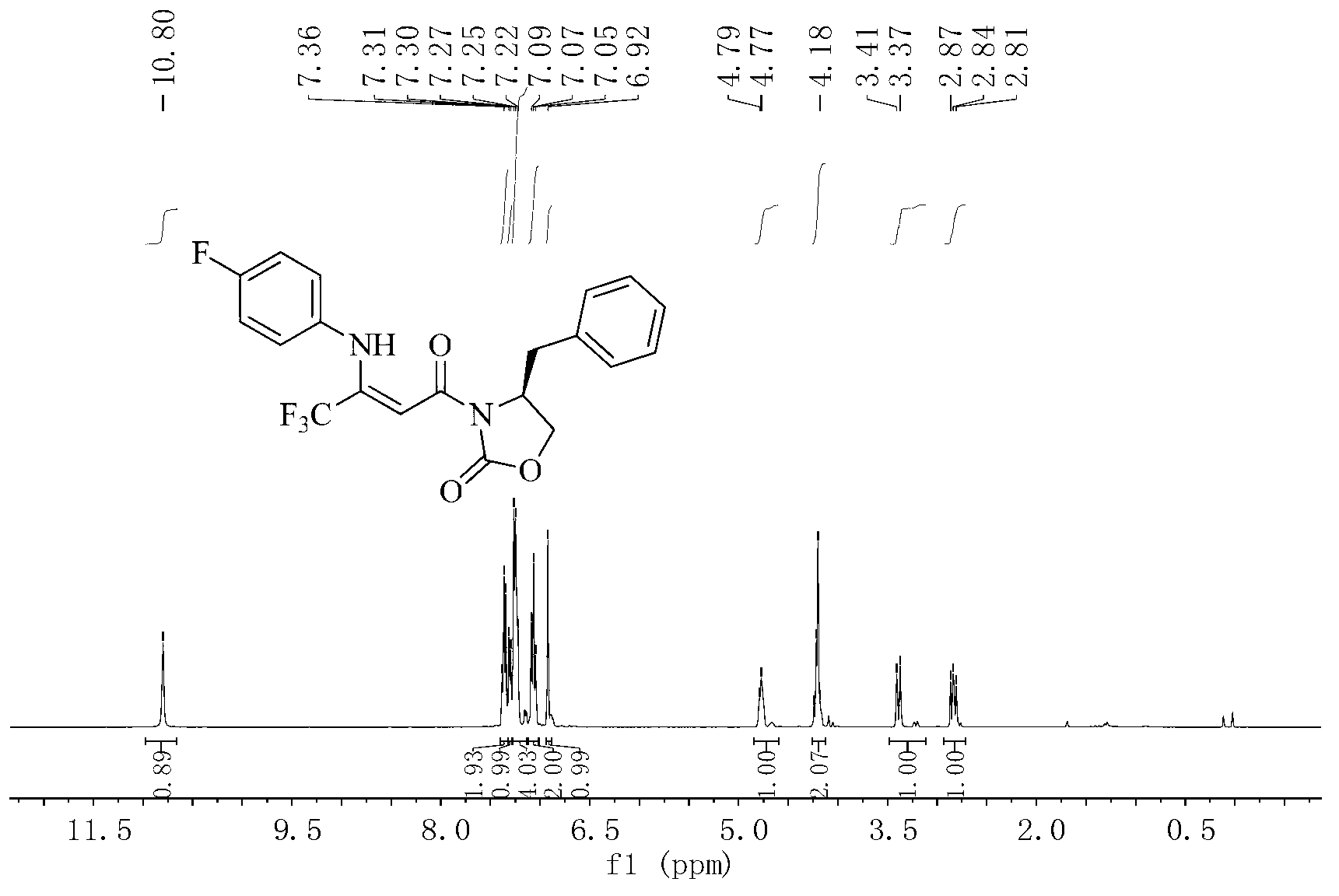
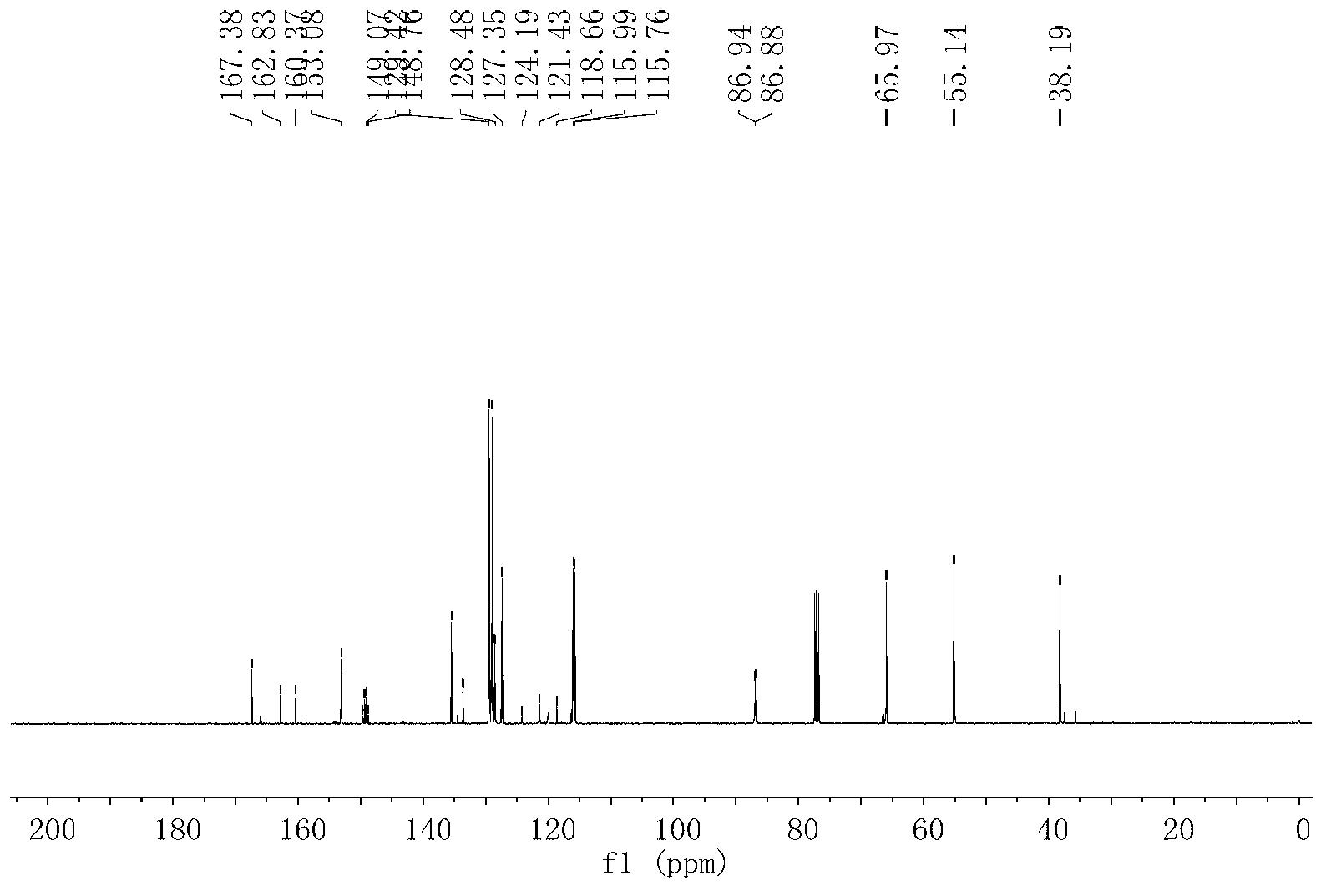
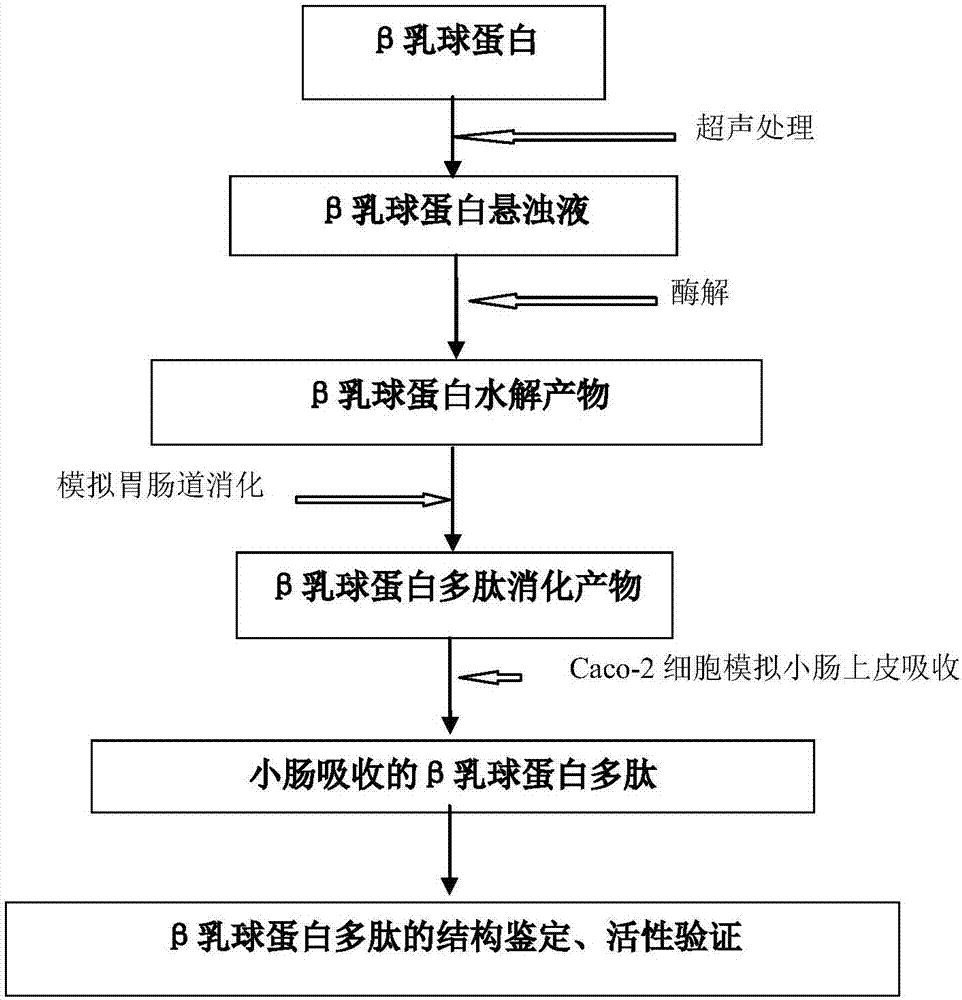
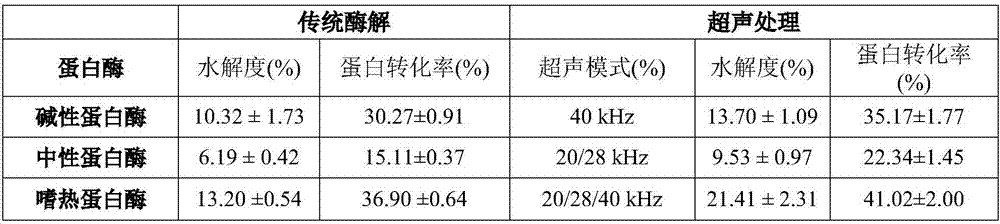
Ultrasonic sound assistant method for simulated digestion of lactoglobulin active peptides and functional food applications
ActiveCN107964040AGood anti-inflammatory activityFermentationAnimals/human peptidesIntestinal structureVascular endothelium
The invention discloses an ultrasonic sound assistant method for simulated digestion of lactoglobulin active peptides and functional food applications, and belongs to the technical fields of dairy product deep processing and functional food preparation. Ultrasonic pretreatment of beta-lactoglobulin is firstly employed; enzymatic hydrolysis is carried out with protease in order to prepare beta-lactoglobulin anti-inflammatory peptides; activity of the beta-lactoglobulin anti-inflammatory peptides is traced by means of simulation of gastrointestinal tract digestion, and after Caco-2 cells simulate absorption of small intestine epithelial cells, the beta-lactoglobulin anti-inflammatory peptides with high anti-inflammatory activity which are digested by gastrointestinal tracts and absorbed by small intestine inner walls which are simulated by Caco-2 cells are represented. Two kinds of beta-lactoglobulin functional polypeptides with high anti-inflammatory activity which are digested by stomach and intestine and absorbed by small intestine inner walls which are simulated by Caco-2 cells are firstly identified; beta-lactoglobulin hydrolysate has good anti-inflammatory activity for blood vessel endothelial cells is firstly reported.
Owner:JIANGSU UNIV
Application of preclinical pharmacokinetic key technology and research system in cefoperazone sodium and sulbactam sodium
The invention provides application of a preclinical pharmacokinetic key technology and research system in cefoperazone sodium and sulbactam sodium, comprising the steps of (1) after the cefoperazone sodium and sulbactam sodium with a certain concentration is administrated to an experimental animal for a certain time, collecting one or more biological samples in blood, urine and faeces; (2) treating the biological samples obtained in step (1) by adopting liquid-liquid extraction, albumen precipitation, and solid phase extraction technologies to obtain corresponding solutions; (3) analyzing the prepared solutions in step (2) by adopting an LC-MS(liquid chromatography-mass spectrography) and an LC-MS / MS((liquid chromatography-tandem mass spectrometry). According to the application provided by the invention, the Caco-2 cell can also be adopted to detect the membrane permeability of the medicine and cell absorptive capacity; or the medicine with a certain concentration treats primary culture cerebral microvascular endothelial cells to detect the blood-brain barrier permeability of the medicine; or by adopting a whole animal, S9, human intestinal microsome and monoclonal purified enzyme, in vitro metabolism stability of the medicine is detected and a metabolite is identified.
Owner:刘晓东 +1
Application of vacuolar protein sorting 4A to preparing medicines for preventing or treating enterovirus type 71 infection
The invention provides application of vacuolar protein sorting 4A (VPS4A) to preparing medicines for preventing or treating enterovirus type 71 infection, relates to the technical field of biomedicine, and provides novel enterovirus type 71 infection resisting targets and application. The application includes that expression of host proteins of target cells which are human colon cancer cells (Caco-2) is down-regulated by the aid of an RNA (ribonucleic acid) interference technology, so that host factors capable of effectively inhibiting EV71 (enterovirus type 71)-infected human colon cancer cells (Caco-2) can be searched, and the purpose of effectively eliminating EV71 infection from sources (intestinal tracts) can be achieved. The application has the advantages that as discovered via experiments, important effects can be realized by the vacuolar protein sorting 4homolog A (VPS4A) for the EV71-infected Caco-2, expression of the VPS4A is down-regulated, and accordingly EV71 infection can be obviously inhibited.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of wedelolactone in preparing drug for resisting ulcerative colitis
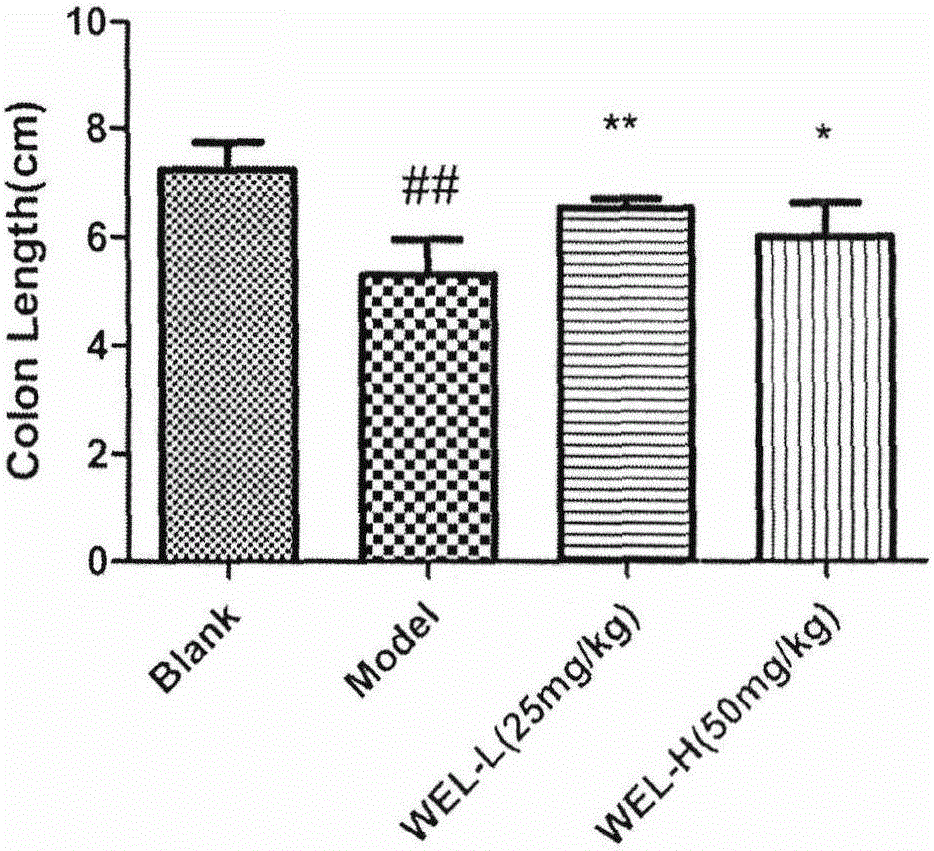
InactiveCN105030763AImprove colitis symptomsGood treatment effectDigestive systemHeterocyclic compound active ingredientsInflammatory factorsWedelolactone
The invention relates to an application of Chinese traditional herb monomer wedelolactone as a drug for treating ulcerative colitis. According to the application, wedelolactone is obtained from Chinese traditional herb material eclipta, and the structure of wedelolactone is determined according to spectrum data. Through intragastric administration of wedelolactone, the body weight change of a model mouse is obviously changed, the length of the colon is increased, the inflammation level of the colon is obviously hanged, the NO content reflecting the inflammation degree of the colon is reduced, the activity of myeloperoxidase (MPO) in the colon tissue is reduced, release of inflammatory factors IL-8 of Caco-2 cells excited byIL-1beta is inhibited in vitro. The results of in-vivo and in-vitro experiments show that wedelolactone under certain dosage can obviously inhibit release of the inflammatory factors of the colon tissue, so that the acute ulcerative colitis of the mouse caused by dextran sulphate sodium salt (DSS) can be obviously improved, and therefore, wedelolactone has a novel application as a drug for treating or improving ulcerative colitis.
Owner:CHINA PHARM UNIV
Method for evaluating biosecurity of 76 nm nano silver on base of intestinal epithelial cells
The invention provides a method for evaluating the biosecurity of 76 nm nano silver on the base of intestinal epithelial cells. According to the method, cell lines of human colon carcinoma epithelial cells Caco-2 are selected as an object of study, nano zinc oxide (90.81 nm) with higher cytotoxicity is taken as a positive control, normal cells without the nano material are taken as a blank control, and the cytotoxicity of the nano material is preliminarily explored by an AO / EB double staining method and a CCK-8 method. The oxidative damage effect and the possible mechanism of nano silver on cells are studied by an oxidative stress method through detecting the influence of emission of ROS, SOD and GSH in the cells Caco-2. The result shows that 76.29nm silver particles (smaller than 200 mu g / ml) don't influence the activity of the cells Caco-2 obviously and cause no oxidative stress damage on the cells Caco-2, but the oxidation resistance of the cells is enhanced and is up to the highest level when the concentration of nano silver is 50 mu g / ml.
Owner:CHINA JILIANG UNIV
Combined evaluation model for bioavailability and toxicity of arsenic in foods on human body
InactiveCN105624039AEvaluate sensitive and efficientMicrobiological testing/measurementVertebrate cellsHuman bodyHuman intestinal absorption
The invention provides a combined evaluation model for bioavailability and toxicity of arsenic in foods on a human body. The model comprises a Caco-2 cell model, a QSG7701 liver cell model and a container which comprises a top side chamber and a substrate side chamber which are separated by a porous membrane, wherein a Caco-2 cell is cultured in the top side chamber, and a QSG7701 liver cell is cultured in the substrate side chamber. The combined evaluation model is structurally similar to the structure of a human body intestinal absorption, can be used for simulating the absorption efficiency of a human body on arsenic in foods and sensitively and efficiently evaluating the toxicity of arsenic in foods, and can be widely applied to food safety evaluation of arsenic in foods.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com