Two series major vault protein of glucagon-like peptide-2 of people and preparation method thereof
A glucagon and protein technology, applied in the direction of glucagon, peptide/protein components, animal/human proteins, etc., can solve the problems of unclear molecular mechanism, tumor-promoting effect on GLP-2 and its analogs, etc. , to achieve the effect of promoting proliferation, convenient preparation, and prolonging action time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0070] The present invention will be described in detail through specific examples below.
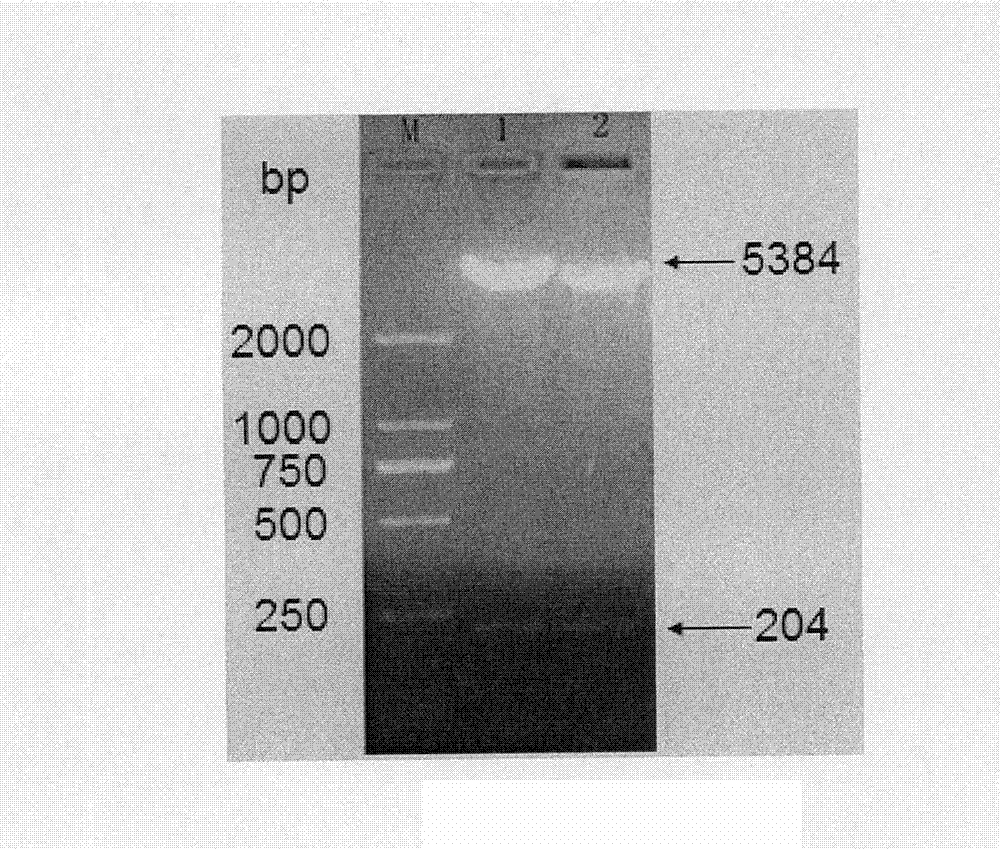
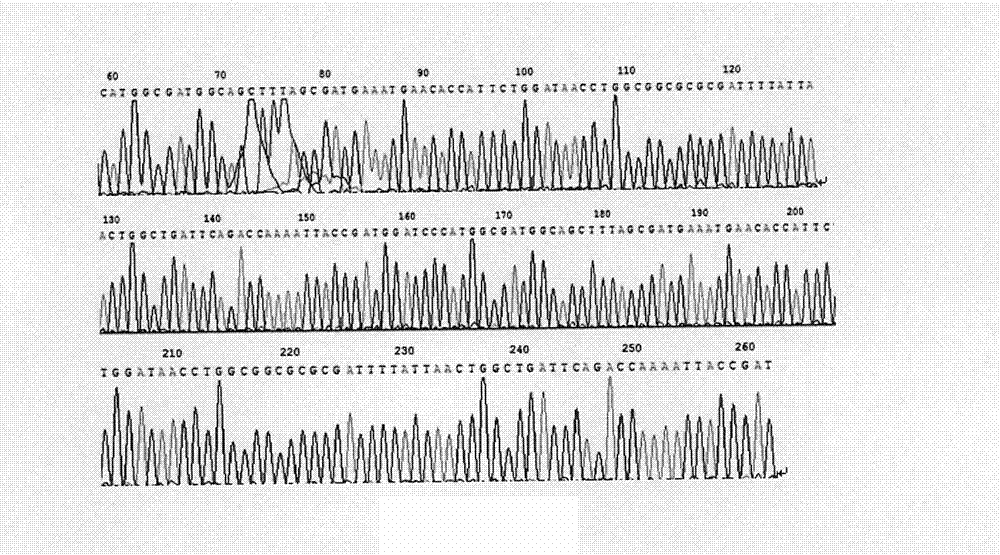
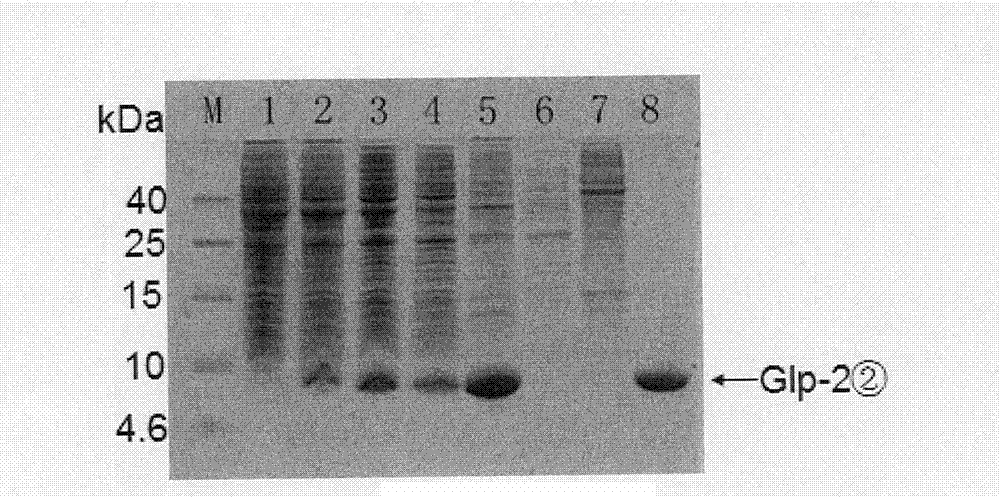
[0071] According to the technical scheme adopted in the present invention, the human glucagon-like peptide-2 dyad gene is synthesized by using the preferred codons of Escherichia coli, and the human glucagon-like peptide-2 dyad gene expression vector pET-22b is constructed. (+)-GLP-2②, the recombinant plasmid was transformed into Escherichia coli BL21 competent cells, and after IPTG induced expression, the recombinant protein was purified by salting out and ion exchange chromatography, and finally a high-purity human glucagon-like peptide was obtained- 2 dimer protein, and use Western blot and HPLC to identify it. The biological activity was determined by Caco-2 cell proliferation assay. The results showed that human glucagon-like peptide-2 dimer protein can promote the proliferation of Caco-2 cells, and its ability to promote the proliferation of Caco-2 cells is higher than that of ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com