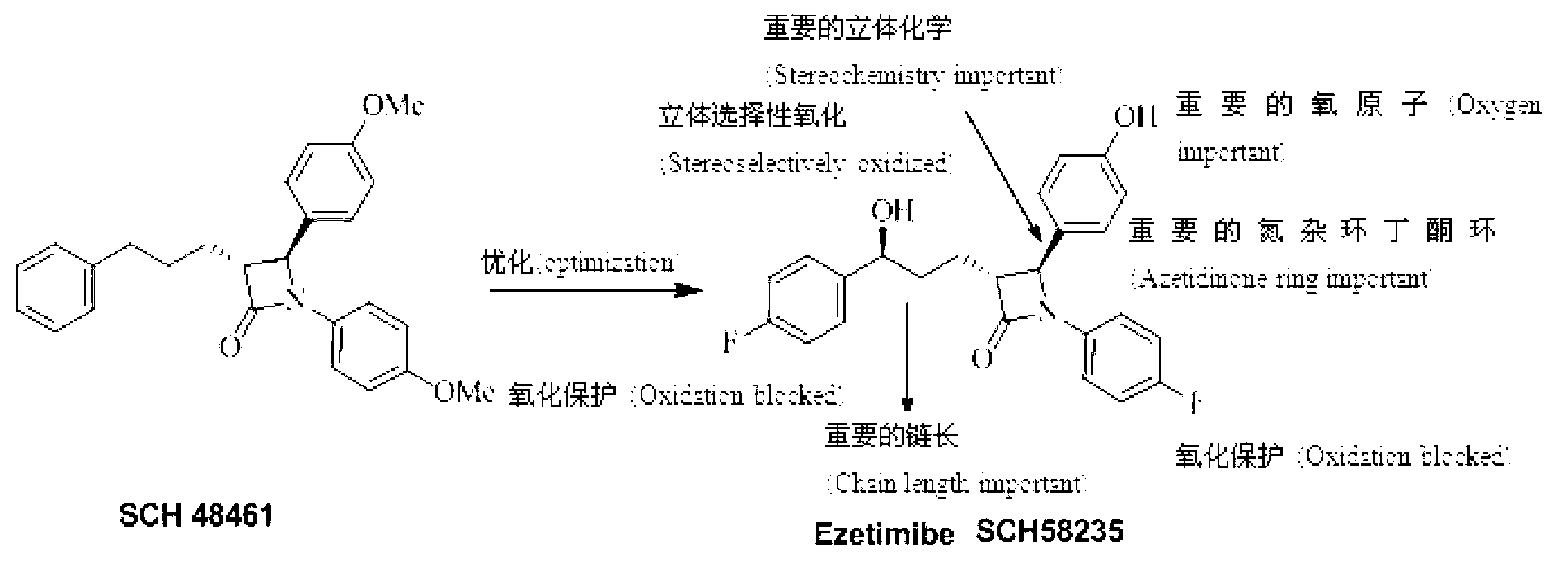
Ezetimibe analogue and preparation method thereof
A technology for ezetimibe and analogs, applied in the field of ezetimibe analogs and their preparation, to achieve the effects of inhibiting absorption and increasing permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Synthesis of intermediate 3d (2,2,2-trifluoro-N-(4-fluorophenyl)acetimidoyl chloride):
[0038]
[0039] Add Ph 3 P (33mmol, 8.655g), Et 3 N (13.2mmol, 1.825mL), CCl 4 (55mmol, 5.275mL) and trifluoroacetic acid (11mmol, 1.254g), after the reaction bottle was stirred in an ice bath for 10 minutes, p-fluoroaniline (11mmol, 1.221g) was added to the reaction bottle with CCl 4 (55mmol, 5.275mL) solution, the mixture was reacted under reflux for 3h, the solvent was spin-off under reduced pressure, the solid mixture obtained was washed with n-hexane and filtered, and the filtrate was concentrated and distilled under reduced pressure to obtain product 3d, ((E) -4-fluoro-N-trifluoroacetal) aniline, the NMR data of this compound are consistent with those reported in the literature (J.Org.Chem.1993, 58, 32).
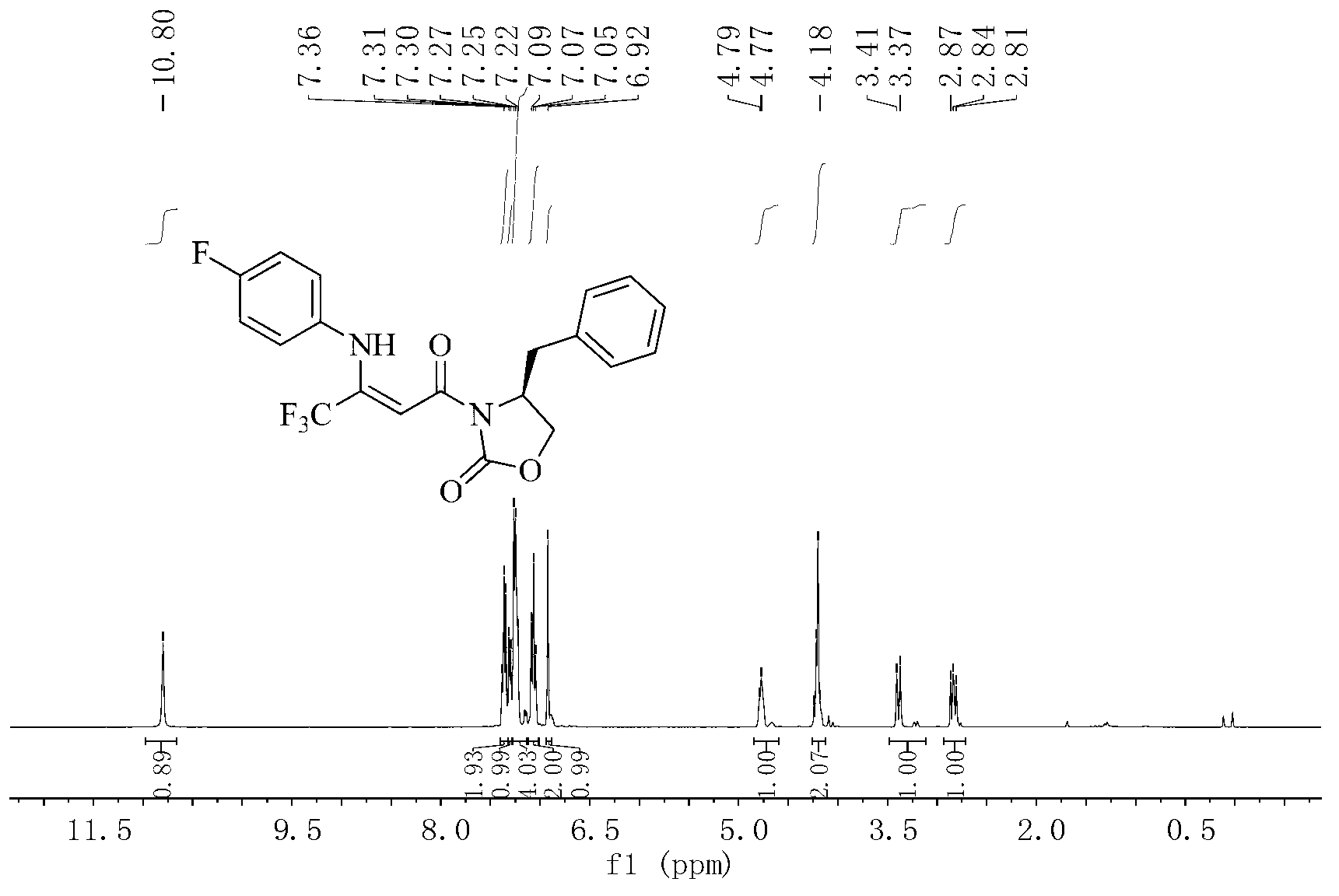
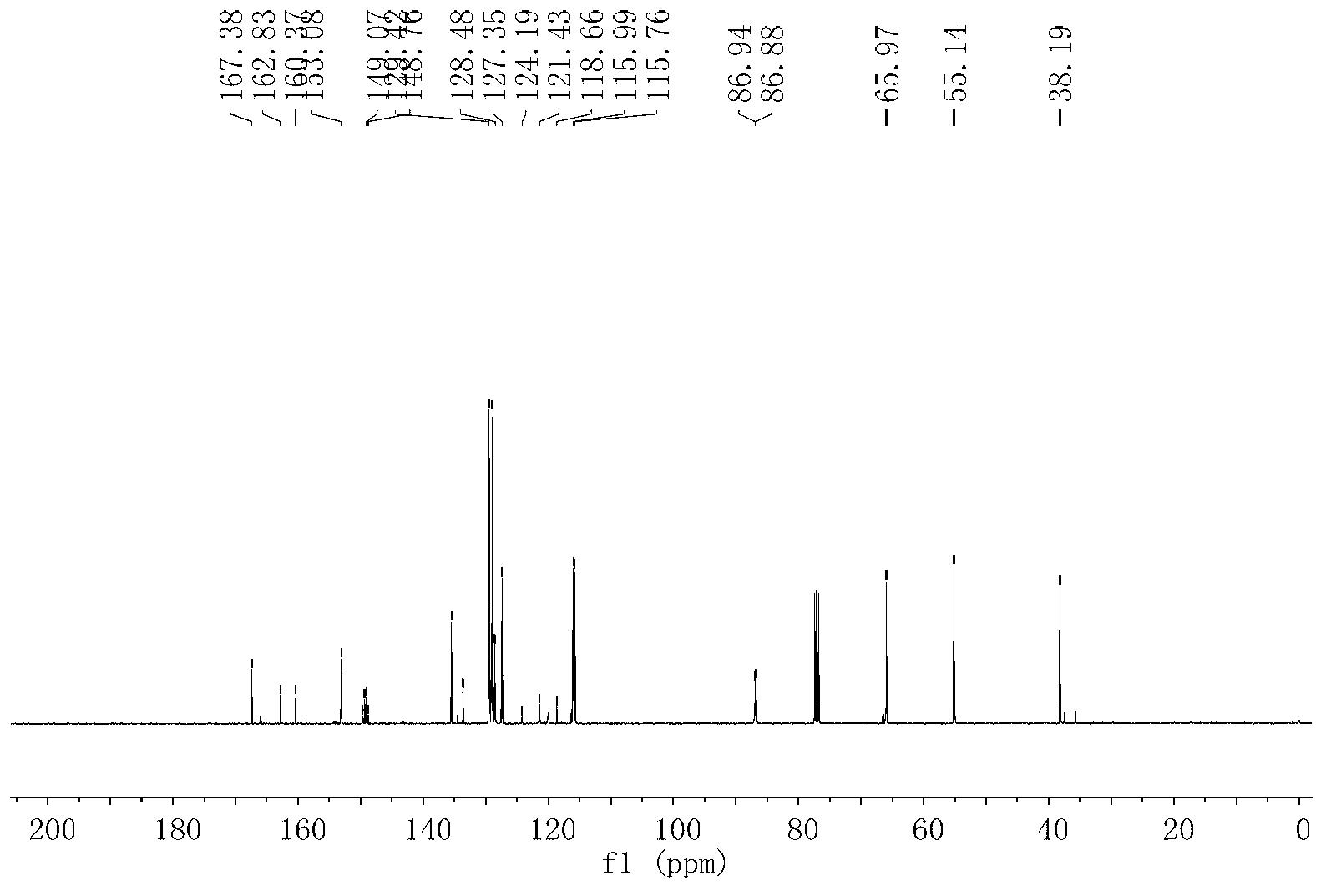
[0040] 2. Synthesis of intermediate 5d:
[0041] (S,Z)-4-benzyl-3-(4,4,4-trifluoro-3-(4-fluorophenylamino)but-2-enoyl)oxazolidin-2-one(5d).
[0042]
[0043]A...
Embodiment 2
[0071] 1. Inhibitory activity of Ezetimibe analogues on the absorption of exogenous cholesterol at the cellular level.
[0072] Tested cells: Caco-2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com