Novel population of hepatocytes derived via definitive endoderm (de-hep) from human blastocysts stem cells
a technology of endoderm and blastocysts, which is applied in the field of new hepatocyte population derived via definitive endoderm (dehep) from human blastocysts stem cells, can solve the problems of large death rate, limited efficacy of this procedure, and major burden on the health care system, and achieves the effect of increasing the percentag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Starting Material
[0220]The starting material for the present invention is suitably pluripotent undifferentiated hBS cells, such as undifferentiated hBS cell lines. Such material can be obtained from Cellartis AB, www.cellartis.com. Cellartis AB is the largest source of defined hBS cell lines world wide and has more than 30 hBS cell lines and subclones available today. Two of the cell lines are listed in the National Institutes of Health (NIH) Human Embryonic Stem Cell Registry, http: / / stemcells.nih.gov / research / registry / and 20 in the UK Stem Cell Bank. Additionally, Cellartis can provide hBS cell lines approved for research use by major markets such as Japan, Germany and France. For the present invention hBS cell line SA002, SA002.5, SA034, SA167, SA181, SA348 and SA461 were used specifically. Characteristics of the hBS cells recommended as starting material are the following: positive for alkaline phosphatase, SSEA-3, SSEA-4, TRA 1-60, TRA 1-81, Oct-4, negative for SSEA-1, telomer...
example 2
Inducing Undifferentiated hBS Cells into DE and Further into Functional Hepatocytes
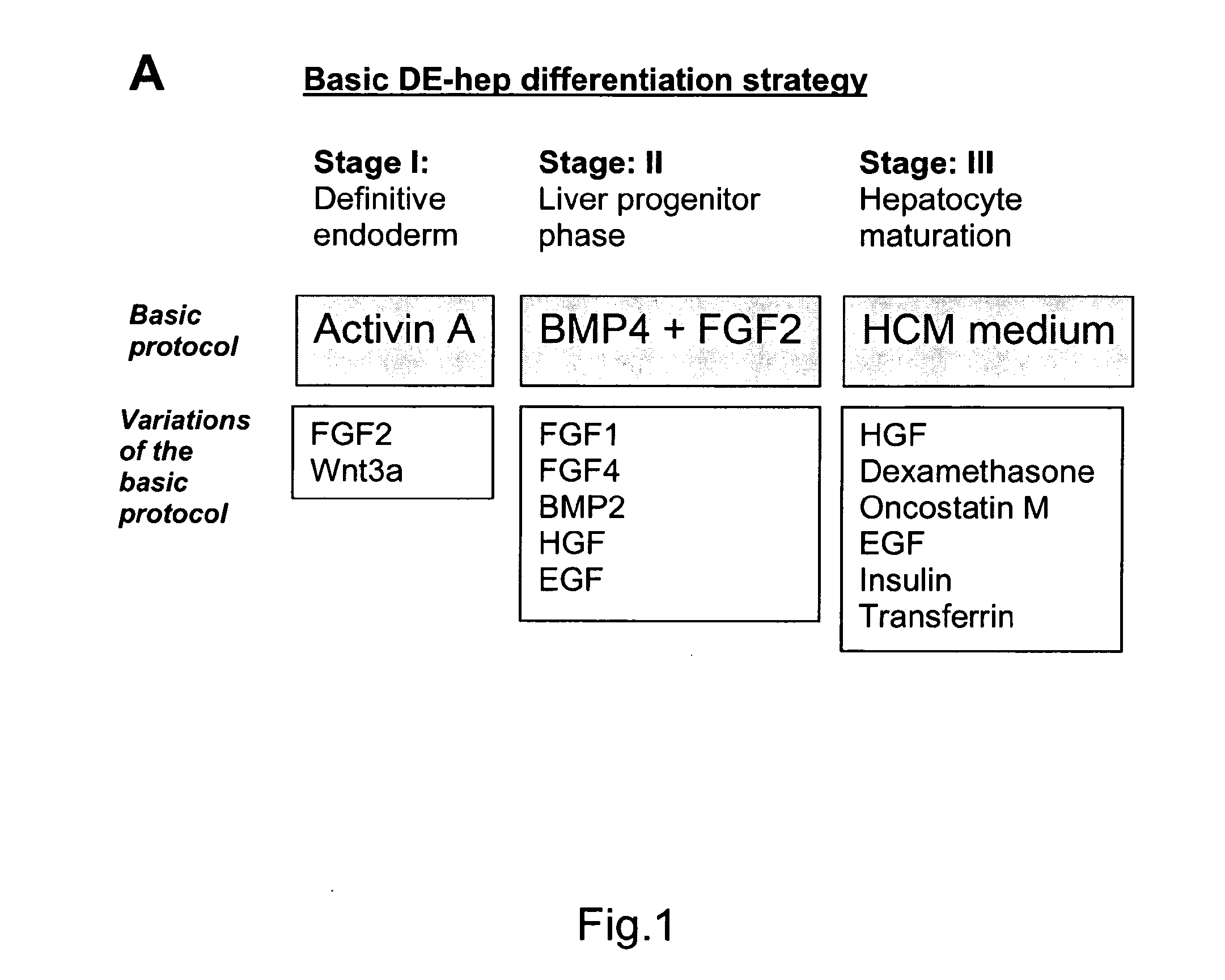
[0224]Undifferentiated hBS cells were maintained and propagated in vitro. At day 4-7 after passage undifferentiated hBS cell colonies were treated according to a basic differentiation program. Each step in this protocol is characterized by key components that are crucial;
Stage I, Definitive endoderm (DE): low serum and Activin A
Stage II, DE-Hep progenitor cells: members of the TGFbeta superfamily of proteins, especially BMP4
Stage III, functional DE-Hep cells: HGF
Changes in growth factor exposure time, concentration, various combinations of them and length of treatment have been tested
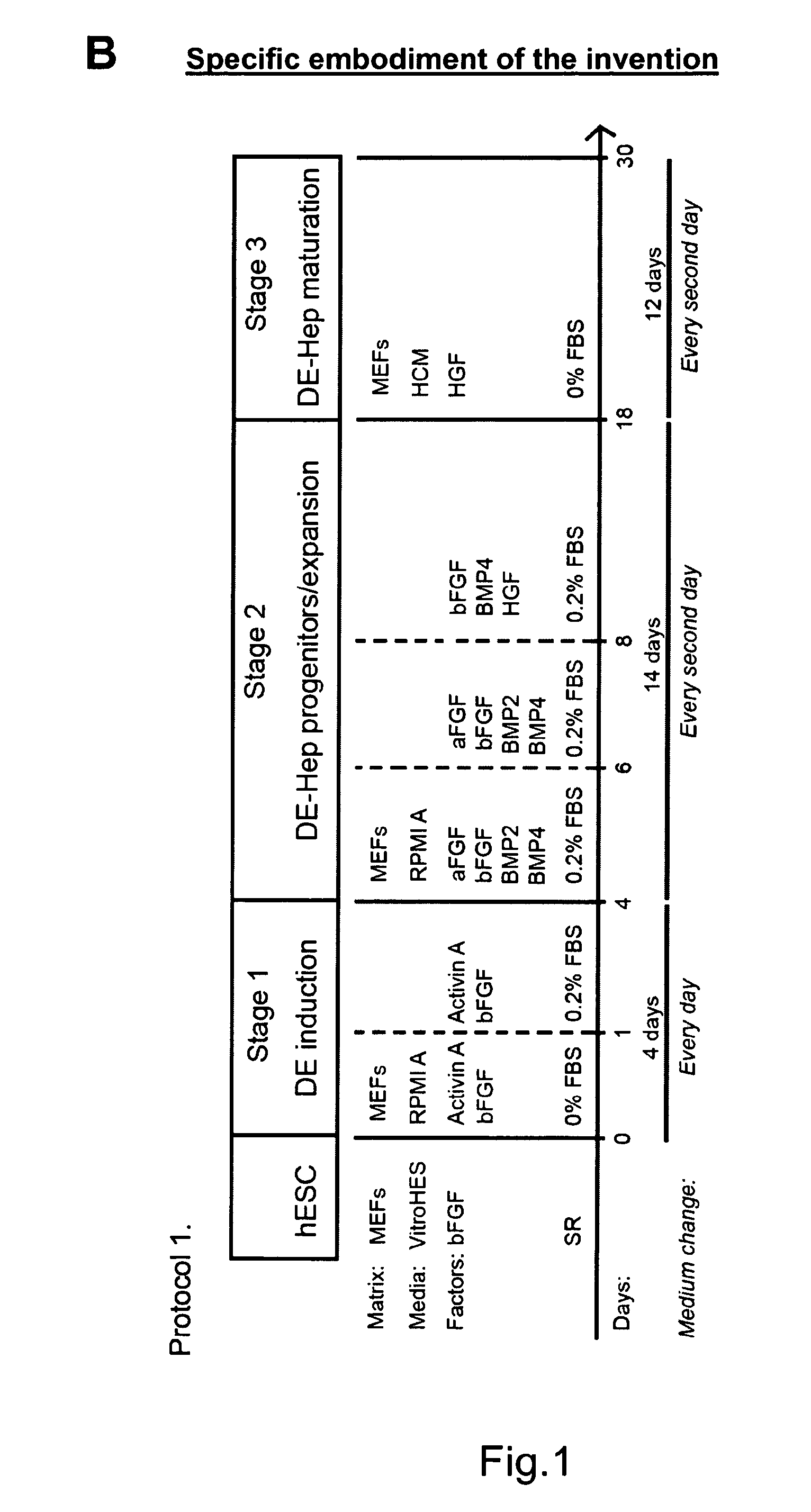
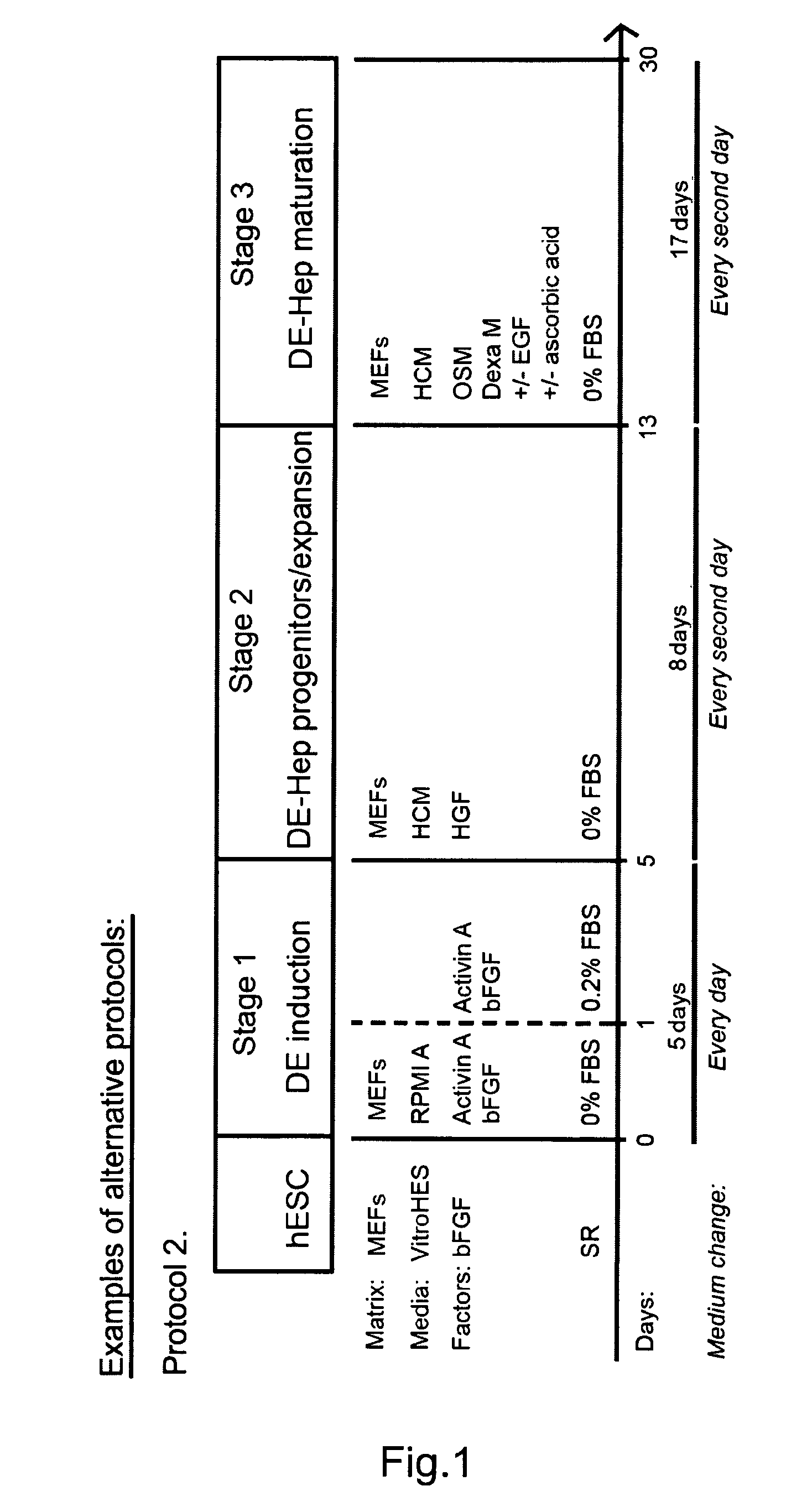
[0225]An overview of the basic method and variations is presented in FIG. 1a. The undifferentiated hBS cells are induced to DE by incubation with Activin A (100 ng / ml) for 3-5 days in RPMI Advanced medium or DMEM medium or a comparable medium (stage I, day 0). The medium is changed every day. The first day of Activin A in...
example 3
Morphology of Definitive Endoderm, Stage I
[0265]Activin A treatment was associated to morphological changes and distinct differentiation pattern of the colonies. After 3-5 days, treated by an Activin A-containing medium, cells gathered together and formed a ring of epithelial cells around the colony (FIG. 2). Those homogenous growing cells expanded over the plate and could be passaged to a Mouse feeder layer containing plate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| humidity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com