Preparation method of galactose derivative cationic liposome nanoparticles
A cationic liposome and nanoparticle technology, applied in liposome delivery, genetic material components, medical preparations of non-active ingredients, etc., can solve the problem of low transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
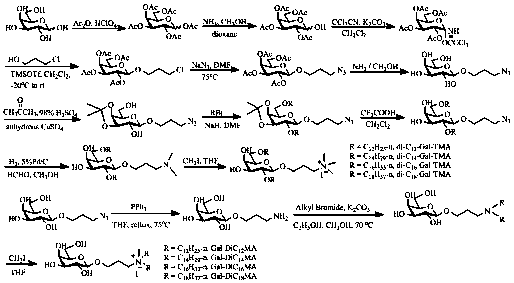
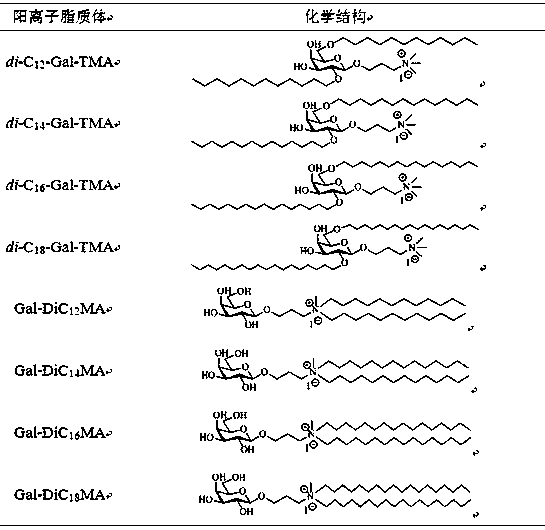
[0033] Embodiment 1. Galactose derivative cationic liposome di -C 12 - Preparation of Gal-TMA nanoparticles:
[0034] Acetic anhydride (393.5 mL, 4.2 mol) was added into a 1.0 L round bottom flask, stirred by magnetic force, and cooled to 0 in an ice bath. o C, add HClO dropwise 4 (2.0 mL). Control temperature is less than 20 o C, add galactose (100.0 g, 0.56 mol) in batches. After the addition, it was naturally raised to room temperature, and was analyzed by TLC (V 石油醚 : V 乙酸乙酯 = 1 : 1) Monitor the reaction. After the reaction is complete, add DCM (200.0 mL) to dilute, wash with water and dichloromethane successively, combine the organic phases, wash with anhydrous MgSO 4 Dry, filter and concentrate to give a white solid. Vacuum drying at room temperature yields 1,2,3,4,6-penta- O - Acetyl-α,β-D-galactopyranose (208.7 g, 96.4%).
[0035] In a 1.0 L round bottom flask add 1,2,3,4,6-penta- O -Acetyl-α,β-D-galactopyranose (79.0 g, 202.4 mmol), mixed with methanol ...
Embodiment 2
[0046] Example 2. Galactose derivative cationic liposomes di -C 14 - Preparation of Gal-TMA nanoparticles:
[0047] In a 100.0 mL round bottom flask, add the compound 3'-azidopropyl 3,4- O -Isopropylidene-β-D-galactopyranoside (1.2 g, 3.9 mmol), was dissolved in anhydrous DMF (30.0 mL), NaH (1.0 g, 23.4 mmol) was added under magnetic stirring, and bromo Tetradecane (4.3 mL, 15.6 mmol). Normal temperature reaction, TLC (V 石油醚 : V 乙酸乙酯 =8:1) Monitor the reaction until the starting material disappears. Extract with water and dichloromethane, combine the organic phases, wash with anhydrous MgSO 4 Dry, filter and concentrate. Through column chromatography (eluent: V 石油醚 : V 乙酸乙酯 = 20: 1) separation and purification to obtain a colorless viscous compound 3'-azidopropyl 2,6-two- O -n-tetradecyl-3,4- O - Isopropylidene-β-D-galactopyranoside (1.5 g, 55.6%). 1 H NMR (500MHz, CDCl 3 ):δ (ppm): 4.19 (d, 1 H, J 1,2 = 8.0 Hz, H-1), 4.11 (dd, 1 H, J 4,3 = J 4,5 = 6....
Embodiment 3
[0052] Example 3. Galactose derivative cationic liposomes di -C 16 - Preparation of Gal-TMA nanoparticles:
[0053] In a 100.0 mL round bottom flask, add the compound 3'-azidopropyl 3,4- O -Isopropylidene-β-D-galactopyranoside (1.2 g, 3.9 mmol), was dissolved in anhydrous DMF (30.0 mL), NaH (1.0 g, 23.4 mmol) was added under magnetic stirring, and bromo Hexadecane (4.8 mL, 15.6 mmol). Normal temperature reaction, TLC (V 石油醚 : V 乙酸乙酯 =8:1) Monitor the reaction until the starting material disappears. Extract with water and dichloromethane, combine the organic phases, wash with anhydrous MgSO 4 Dry, filter and concentrate. Through column chromatography (eluent: V 石油醚 : V 乙酸乙酯 = 20: 1) separation and purification to obtain white solid compound 3'-azidopropyl 2,6-two- O -n-hexadecyl-3,4- O - Isopropylidene-β-D-galactopyranoside (1.9 g, 63.3%). 1 H NMR (500MHz, CDCl 3 ):δ (ppm): 4.21 (d, 1 H, J 1,2 = 8.0 Hz, H-1), 4.12 (dd, 1 H, J 4,3 =6.0 Hz, H-4), 4.06 (t,1 ...
PUM
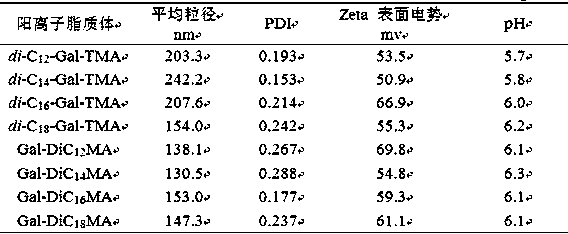
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com