Diagnostic kit for early assessment of risk of adverse reactions to chemotherapy of taxanes
A diagnostic kit and taxane technology, applied in the fields of genetic engineering and oncology, can solve the problems of inaccurate use of Chinese Han population for clinical medication guidance, different gene frequencies, and different SNP loci distribution, etc. Realize individualized treatment guidance, improve efficacy and safety, and quickly and accurately grasp the effect of patients' conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] This embodiment includes sample collection and sorting, genomic DNA sample preparation, genotype detection, statistical analysis and result analysis.
[0021] 1. Sample collection and arrangement
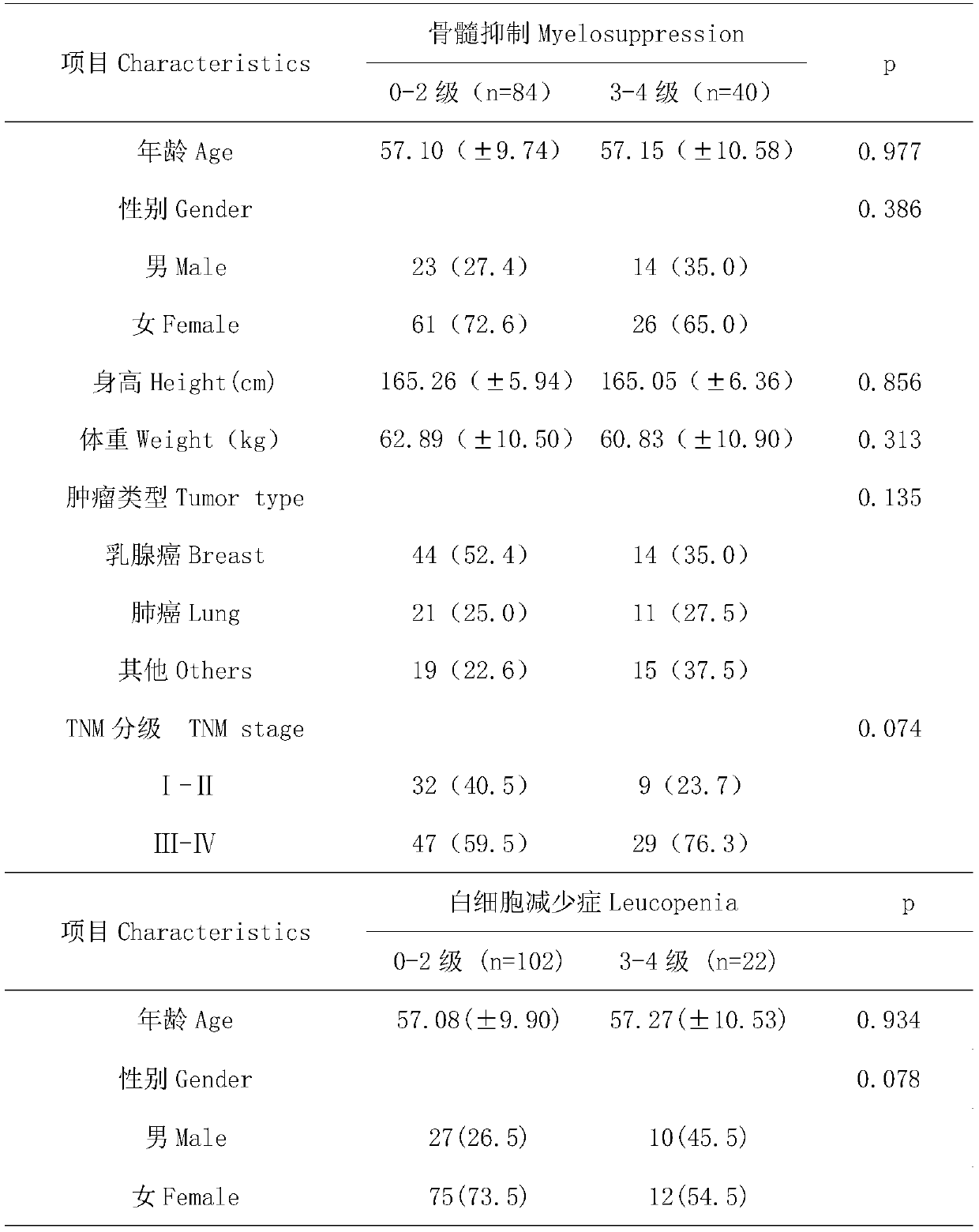
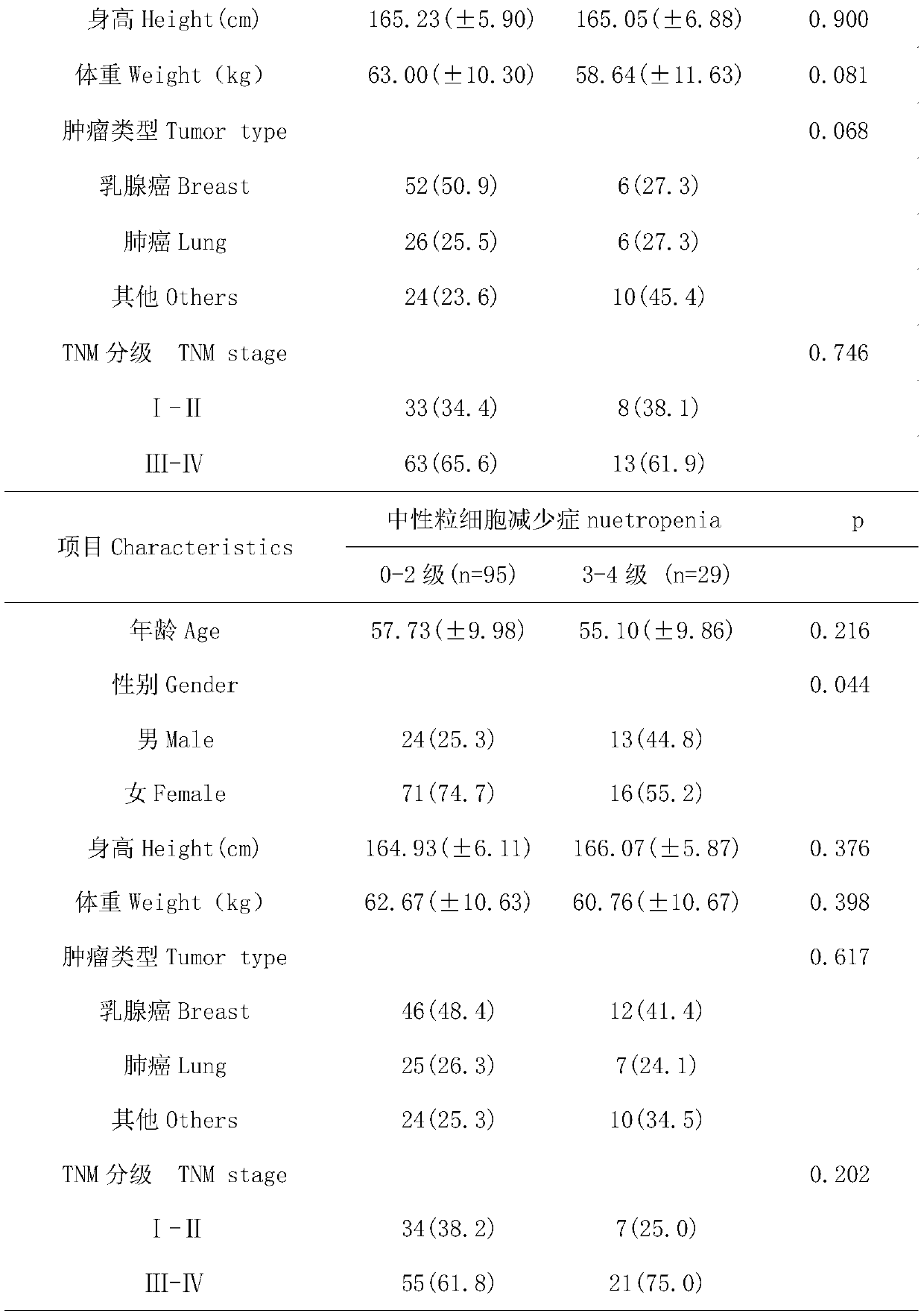
[0022] From 2012 to 2015, a large number of blood samples of cancer patients were collected from the Fourth People's Hospital of Jinan City. After sorting out the sample data, 124 samples meeting the following criteria were selected: (1) cancer patients aged 18 to 80 Patients; (2) no major organ dysfunction, blood routine, liver and kidney function and heart function were basically normal before the first chemotherapy; (3) all patients received at least one cycle of chemotherapy based on paclitaxel or docetaxel; (4) except In patients with bone metastases, all patients had a baseline absolute neutrophil count greater than 1.5×10 9 / L; (5) Baseline information such as age, gender, height, weight, tumor type, and tumor stage of the patient; (6) Detailed information on drug tox...
Embodiment 2
[0040] This embodiment is the application of a diagnostic kit based on PCR technology and DNA sequencing technology.
[0041] 1. DNA extraction
[0042] DNA was extracted from peripheral blood using the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the product instructions, and stored at -20°C.
[0043] 2. PCR reaction
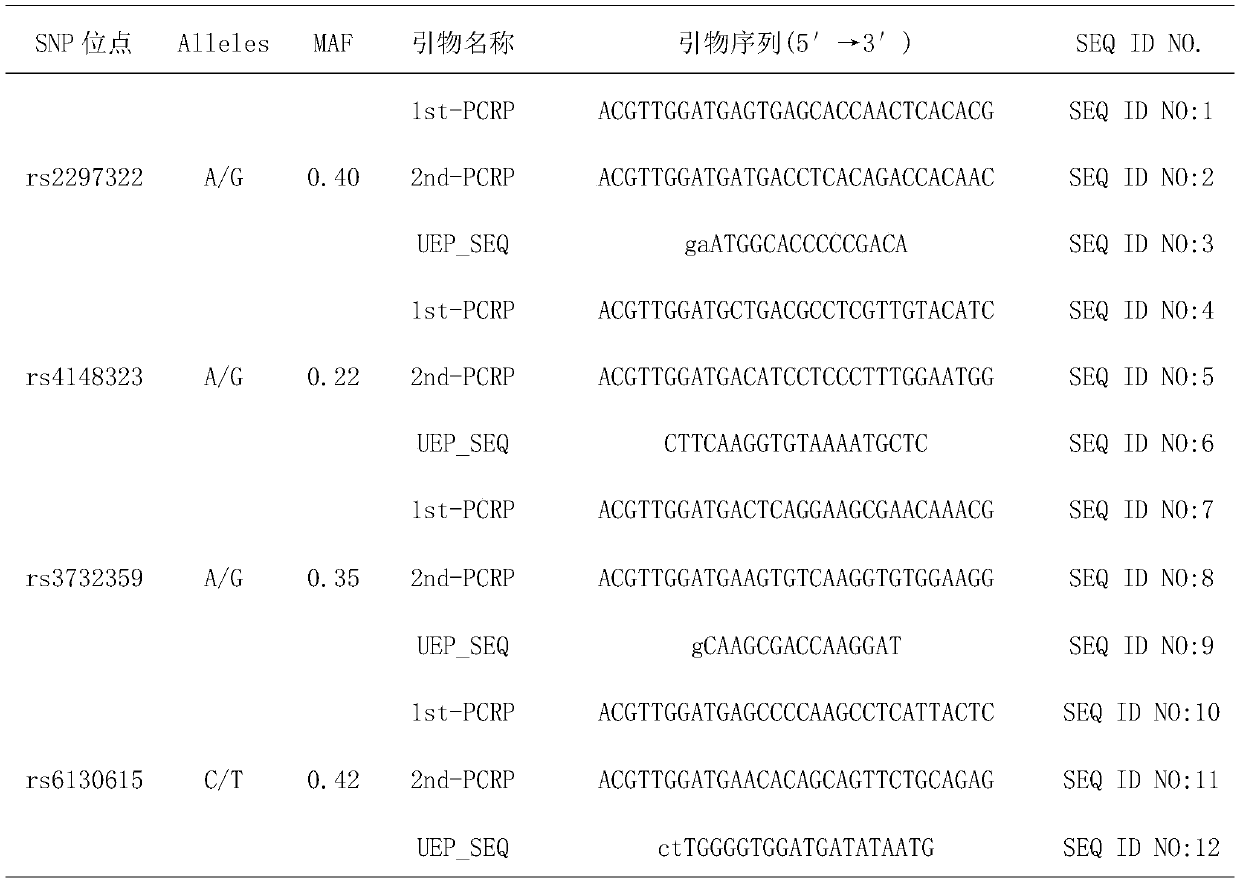
[0044] Use the PCR reaction kit from the diagnostic kit, which contains the following primer pairs:
[0045] The sequence of the front primer of SLC15A1rs2297322 is SEQ ID NO:1, and the sequence of the back primer is SEQ ID NO:2;
[0046] The sequence of the front primer of UGT1A1rs4148323 is SEQ ID NO:3, and the sequence of the back primer is SEQ ID NO:4;
[0047] The sequence of the front primer of NR1I2rs3732359 is SEQ ID NO:5, and the sequence of the back primer is SEQ ID NO:6;
[0048] The sequence of the front primer of HNF4αrs6130615 is SEQ ID NO:7, and the sequence of the back primer is SEQ ID NO:8.
[0049] For details of the ab...
Embodiment 3
[0067] This example provides taxane chemotherapy drug-related myelosuppression risk gene detection services.
[0068] 1. DNA extraction
[0069] Blood samples were collected from the subjects by laboratory physicians in the hospital, and genomic DNA was extracted from whole blood using the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com