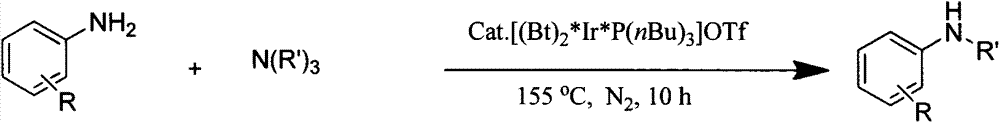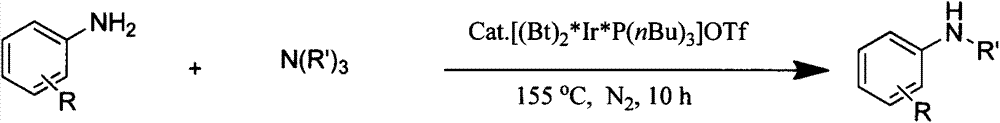Novel method for preparing secondary amine by reaction of primary amine and tertiary amine
A technology for tertiary amines and secondary amines, applied in chemical instruments and methods, formation/introduction of amino groups, preparation of organic compounds, etc., can solve problems such as difficult demethylation of N-methylamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment one: a kind of novel method that obtains secondary amine by reaction of aniline and triethylamine comprises the following process steps:
[0022] Under nitrogen protection, in 50mL Schlenk tube, add aniline (93mg, 1mmol), triethylamine (304mg, 3mmol), [(Bt) 2 *Ir*P(nBu) 3 ]OTf (9.6mg, 0.01mmol, 1.0mol%), add 2.0mL xylene; react the reaction solution at 155°C for 10h, and separate the resulting solution with a 200-300 mesh silica gel column (the eluent is 1:10 ethyl acetate ester / petroleum ether), and the product was obtained after removing the solvent.
[0023] Yield: 95%.
Embodiment 2
[0024] Embodiment two: a kind of novel method that obtains secondary amine by reacting 4-methoxyaniline and triethylamine, comprises the following processing steps:
[0025] Under nitrogen protection, in 50mL Schlenk tube, add 4-methoxyaniline (123mg, 1mmol), triethylamine (304mg, 3mmol), [(Bt) 2 *Ir*P(nBu) 3 ]OTf (9.6mg, 0.01mmol, 1.0mol%), add 2.0mL xylene; react the reaction solution at 155°C for 10h, and separate the resulting solution with a 200-300 mesh silica gel column (the eluent is 1:10 ethyl acetate ester / petroleum ether), and the product was obtained after removing the solvent.
[0026] Yield: 92%.
Embodiment 3
[0027] Embodiment three: a kind of novel method that obtains secondary amine by reacting 4-methylaniline and triethylamine, comprises the following processing steps:
[0028] Under nitrogen protection, in 50mL Schlenk tube, add 4-methylaniline (107mg, 1mmol), triethylamine (304mg, 3mmol), [(Bt) 2 *Ir*P(nBu) 3 ]OTf (9.6mg, 0.01mmol, 1.0mol%), add 2.0mL xylene; react the reaction solution at 155°C for 10h, and separate the resulting solution with a 200-300 mesh silica gel column (the eluent is 1:10 ethyl acetate ester / petroleum ether), and the product was obtained after removing the solvent.
[0029] Yield: 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com