Chlorambucil derivative, and preparation method and application thereof
A technology for chlorambucil and derivatives, which is applied in the directions of pharmaceutical formulations, pharmaceutical combinations, and medical preparations containing active ingredients, etc., can solve the problems of large toxic and side effects, limited therapeutic effect, short half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
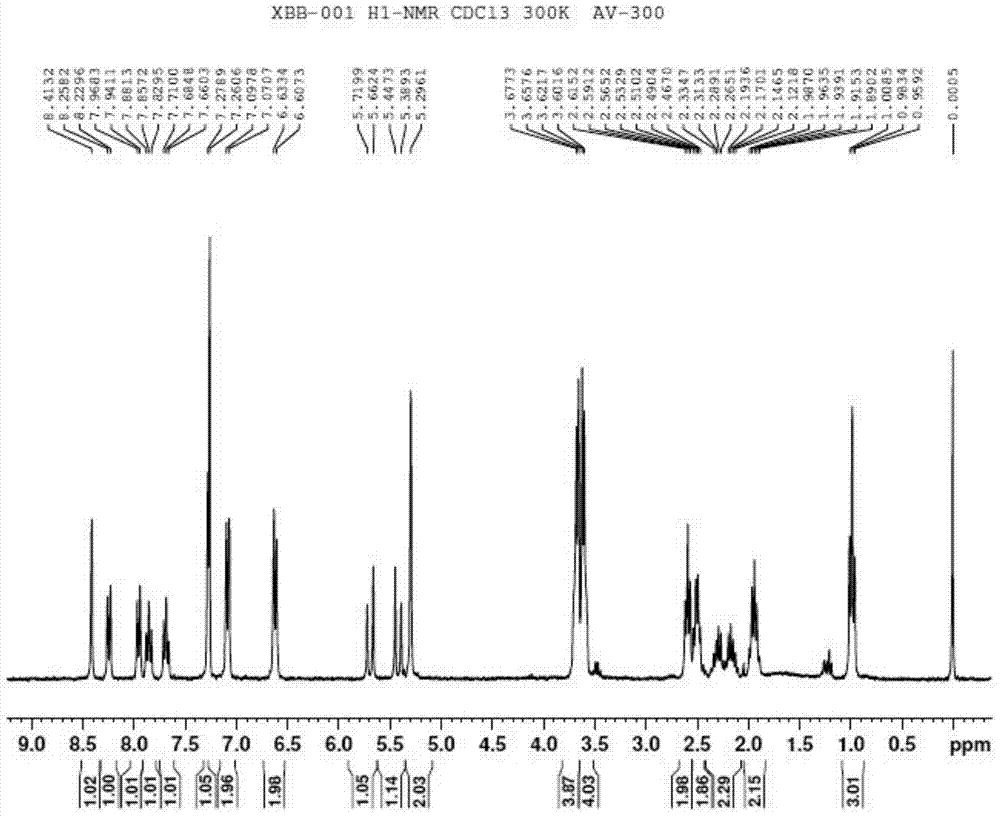
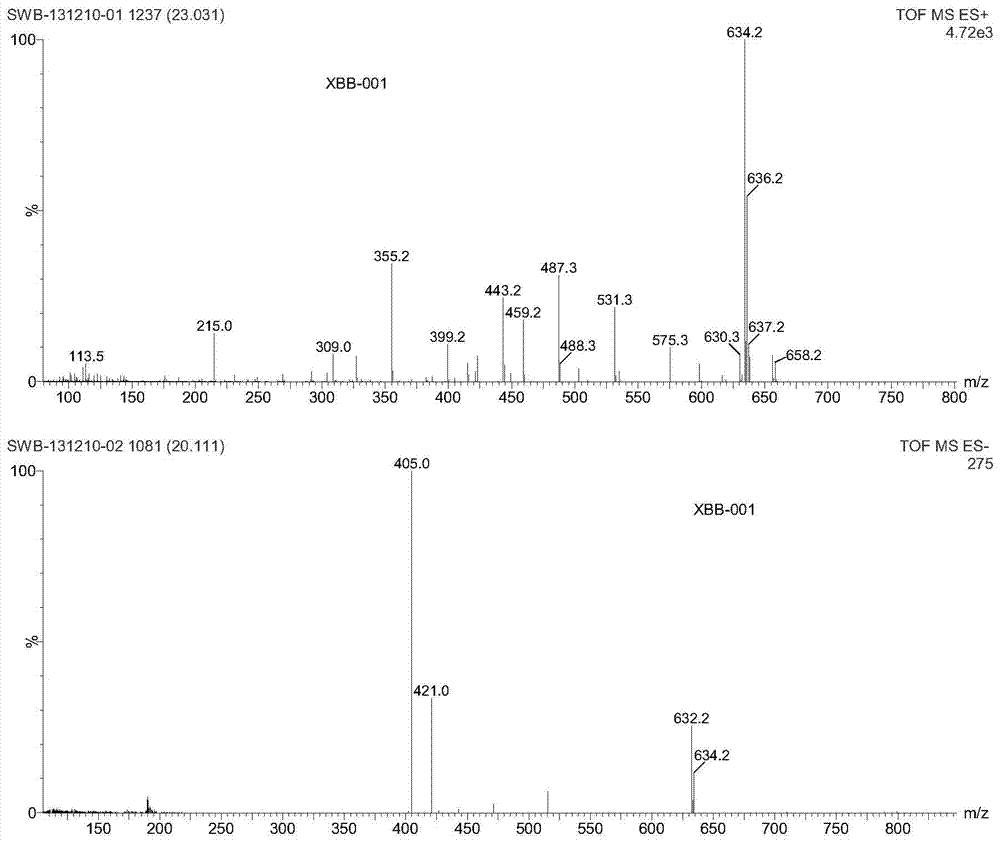
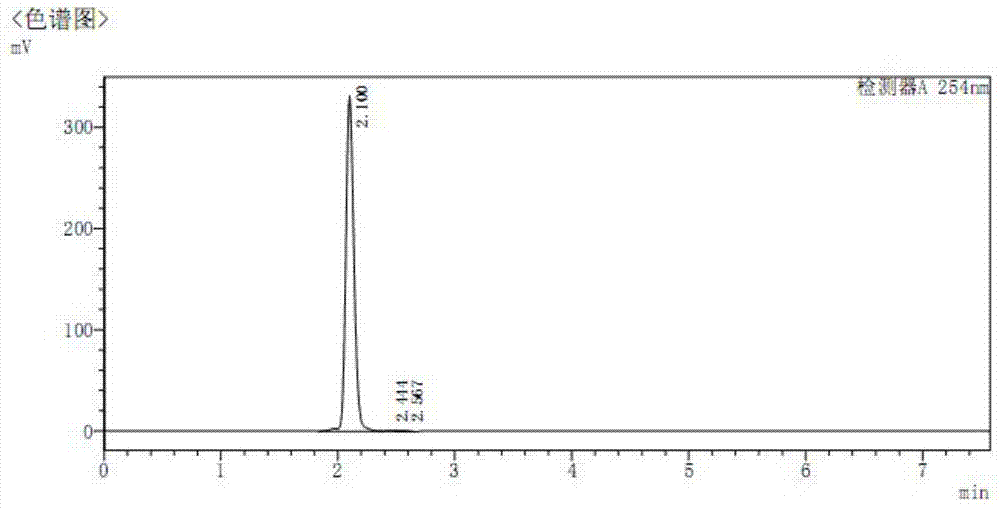
[0121] Example 1. Synthesis of 4-[bis(2-chloroethyl)amino]benzenebutyrate camptothecin ester (XBB-001, compound of formula II, R=H)
[0122] The reaction formula is as follows:
[0123]
[0124] Experimental steps:
[0125] Into a 100 mL round bottom flask, 0.348 g (1 mmol) of camptothecin and 40 mL of anhydrous DMF were added and heated to dissolve.
[0126] 0.334g (1.1mmol) 4-[p-bis(2-chloroethyl)amino]phenylbutyric acid was added to the solution, then 0.501g (2mmol) 2-chloro-1-methylpyridinium iodide, 0.489 g (4 mmol) 4-dimethylaminopyridine, stirred at room temperature, and reacted overnight until the reaction was complete. 200mL ethyl acetate was added to the reaction solution, stirred for 15 minutes, transferred to a separatory funnel, the mixed solution was washed three times with 100mL salt water, the organic phase was dried with 20g anhydrous magnesium sulfate for 50 minutes, filtered to remove magnesium sulfate, and removed by rotary evaporation Solvent ethyl acetate. The...
Embodiment 24
[0130] Example 2. Synthesis of 7-ethyl-10-hydroxycamptothecin (XBB-002, compound of formula I, R is ethyl)-[bis(2-chloroethyl)amino]phenylbutyric acid
[0131] The reaction formula is as follows:
[0132]
[0133] Experimental steps:
[0134] Add 0.456g (1.5mmol) 4-[p-bis(2-chloroethyl)amino]phenylbutyric acid, 30mL anhydrous toluene, 600μL thionyl chloride and 2 drops of anhydrous DMF into a 100mL round bottom flask, protected by nitrogen After stirring for 6 hours at room temperature, the excess thionyl chloride and anhydrous toluene were removed by rotary evaporation under reduced pressure, and then 10 mL of chloroform was added to dissolve the residue to obtain solution A.
[0135] Weigh 0.392 g (1 mmol) of 7-ethyl-10-hydroxycamptothecin and add it to another 100 mL round bottom flask, add 20 mL of anhydrous DMF, heat and stir to dissolve, and add 250 μL of anhydrous triethylamine. The solution A was slowly added dropwise to the above solution through the dropping funnel. The add...
Embodiment 34
[0140] Example 3. Synthesis of 10-[bis(2-chloroethyl)amino]phenylbutyric acid 10-hydroxycamptothecin (XBB-003, compound of formula I, R=H)
[0141] The reaction formula is as follows:
[0142]
[0143] Experimental steps:
[0144] Add 0.456g (1.5mmol) 4-[p-bis(2-chloroethyl)amino]phenylbutyric acid, 30mL anhydrous toluene, 600μL thionyl chloride and 2 drops of anhydrous DMF into a 100mL round bottom flask, protected by nitrogen After stirring for 6 hours at room temperature, the excess thionyl chloride and anhydrous toluene were removed by rotary evaporation under reduced pressure, and then 10 mL of chloroform was added to dissolve the remainder to obtain solution A.
[0145] Weigh 0.364 g (1 mmol) of 10-hydroxycamptothecin and add it to another 100 mL round bottom flask, add 20 mL of anhydrous DMF, heat and stir to dissolve it, and add 250 μL of anhydrous triethylamine. The solution A was slowly added dropwise to the above solution through the dropping funnel. The addition was compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com