Nonaqueous electrolyte secondary battery and method for manufacturing nonaqueous electrolyte secondary battery
A non-aqueous electrolyte, secondary battery technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, non-aqueous electrolytes, etc., can solve problems such as DC resistance rise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
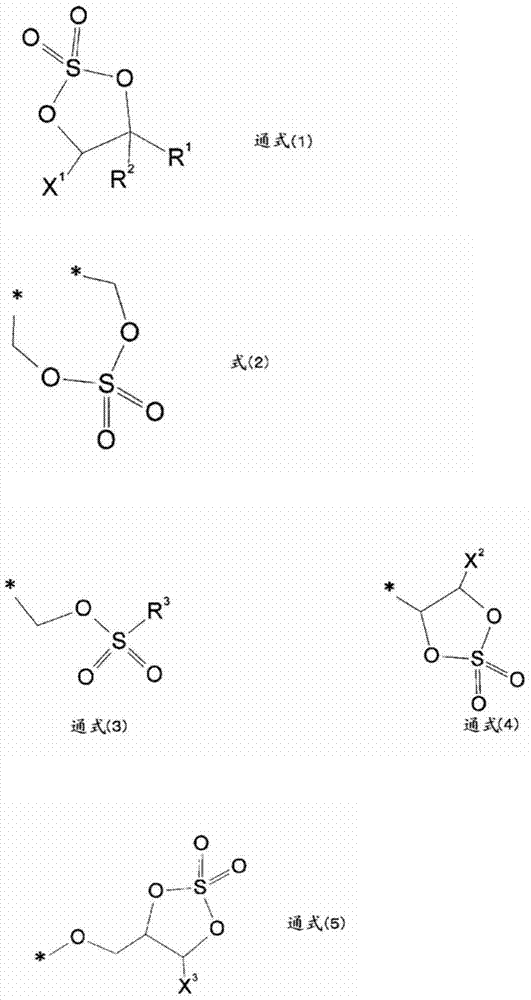
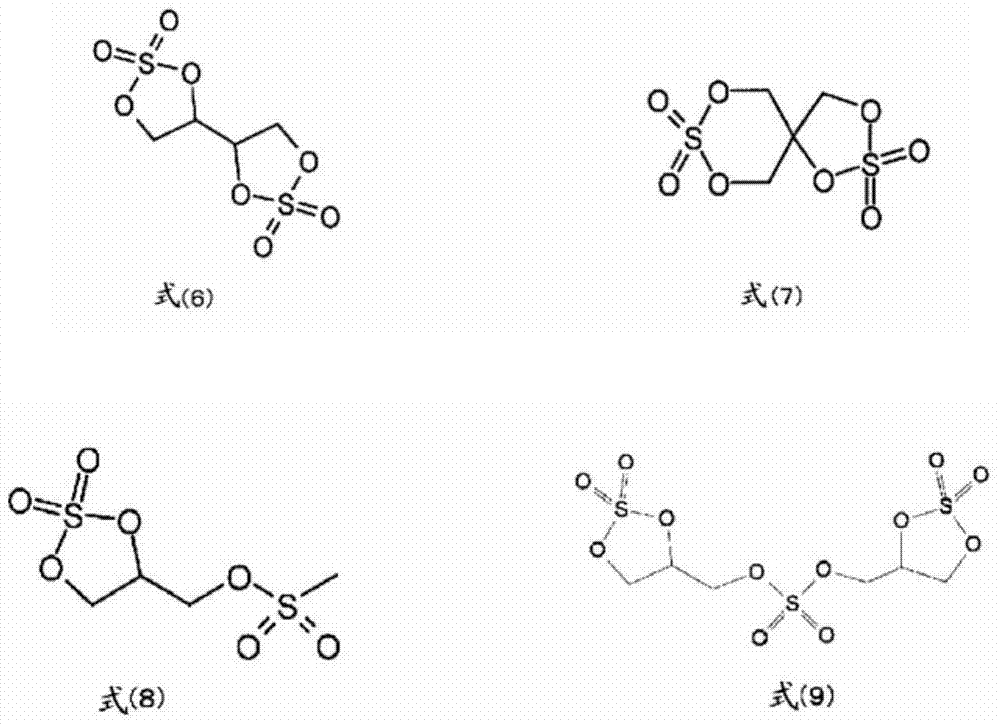
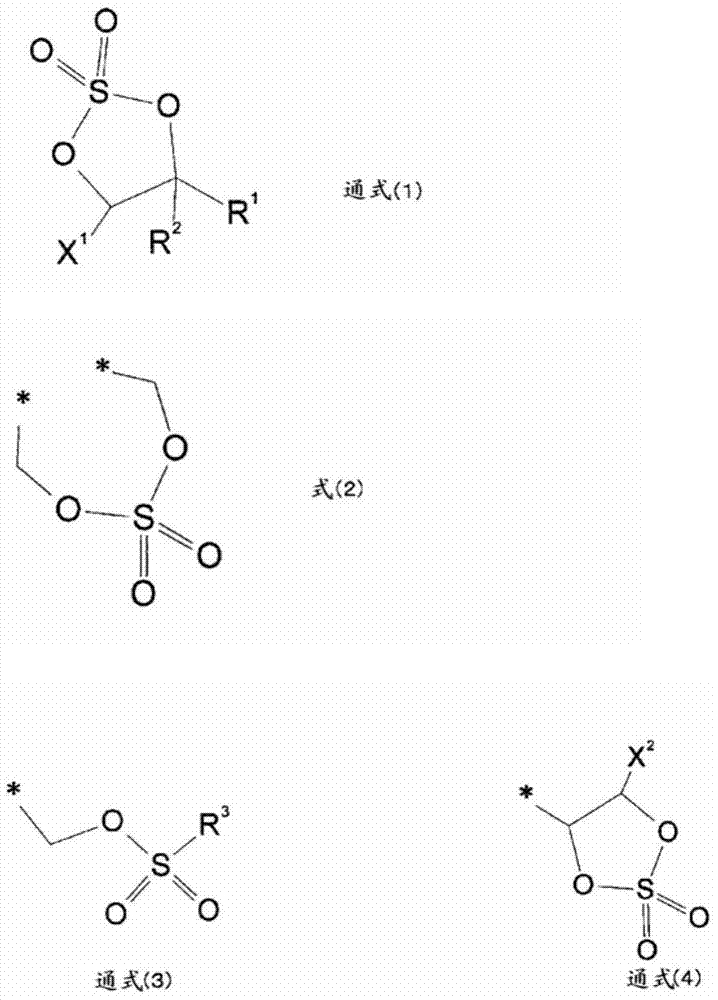
Image
Examples
Embodiment 1
[0051] A lithium ion secondary battery as a nonaqueous electrolyte secondary battery was produced by the methods (1) to (4) shown below.
[0052] (1) Production of positive electrode
[0053] Manganese sulfate hydrate, nickel sulfate hydrate, and cobalt sulfate hydrate were dissolved in ion-exchanged water so that the respective elements of manganese, nickel, and cobalt became 1:1:1, to prepare a mixed solution. At this time, the concentration of the mixed solution was 0.667 M and the volume was 180 ml. Next, 600 ml of ion-exchanged water was prepared in a 1-liter beaker, and 8N NaOH was added dropwise to prepare a lye solution adjusted to pH 11.5. In addition, the temperature of the beaker was adjusted to 50° C., and Ar gas was bubbled in the lye. Thereafter, the above-mentioned mixed solution was added dropwise to the alkali solution at 3 ml / min while maintaining the temperature and pH of the alkali solution. At the same time, 50 ml of 2.0 M hydrazine aqueous solution was...
Embodiment 2~16 and comparative example 1~10
[0061] As shown in Table 1, except for changing the type of the positive electrode active material, the average particle diameter of the positive electrode active material, the type of the cyclic sulfate ester compound, or the concentration of the cyclic sulfate ester compound in the electrolyte, it is the same as in Example 1. to manufacture lithium-ion secondary batteries. In addition, in Comparative Examples 1 to 4, instead of the lithium nickel-cobalt-manganese composite oxide used as the positive electrode active material, Li1.1 mn 1.8 al 0.1 o 4 It is produced by the following general synthesis method. That is, by mixing lithium hydroxide, aluminum hydroxide and MnO in a specified molar ratio 2 The obtained solution was dried by a spray drying method to obtain a precursor containing Li and Mn. The precursor was pre-calcined in air at 500°C for 12 hours, followed by calcination at 750°C for 12 hours, and further crushed and classified to obtain Li with an average part...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com