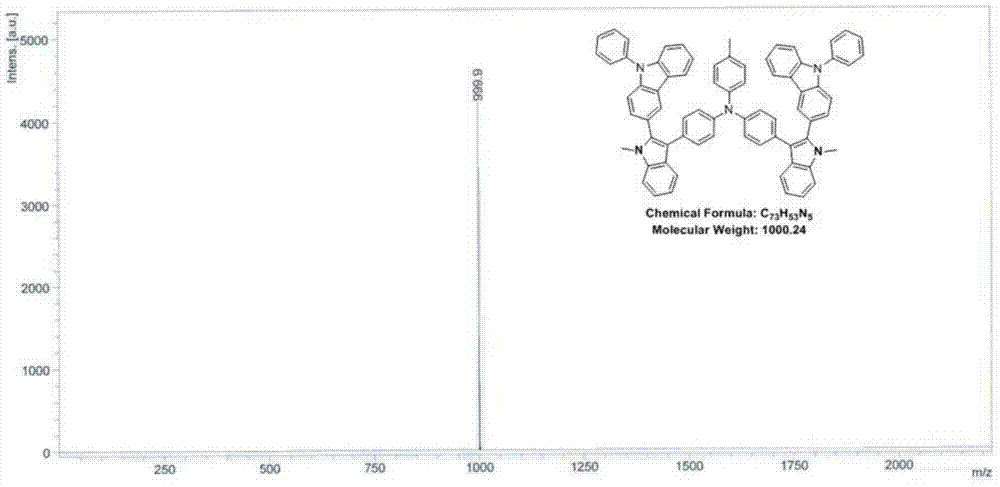
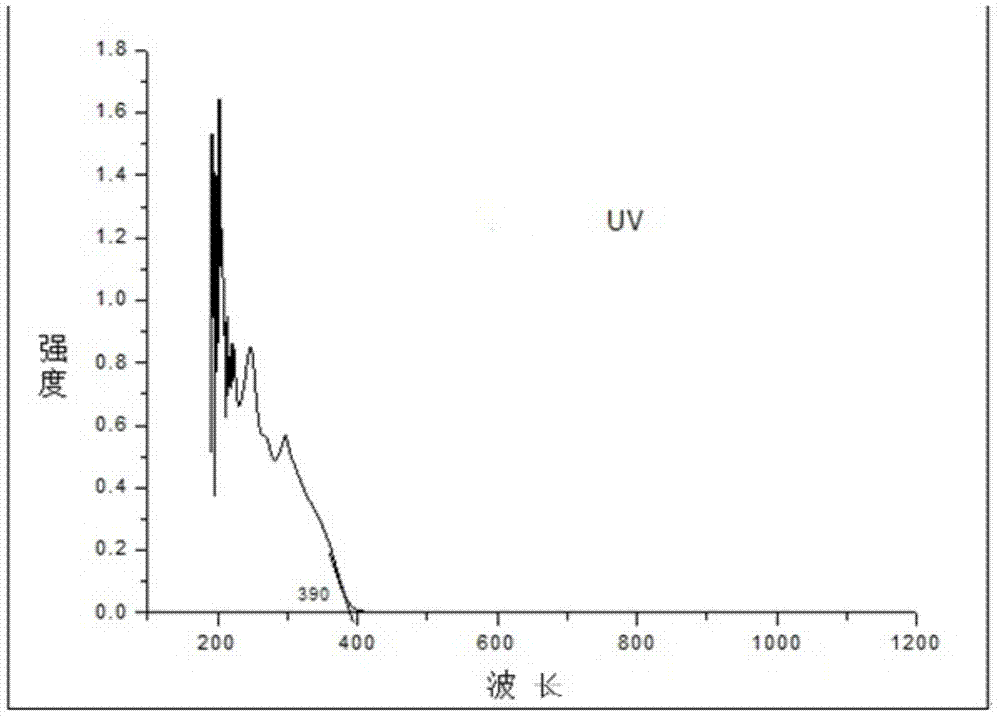
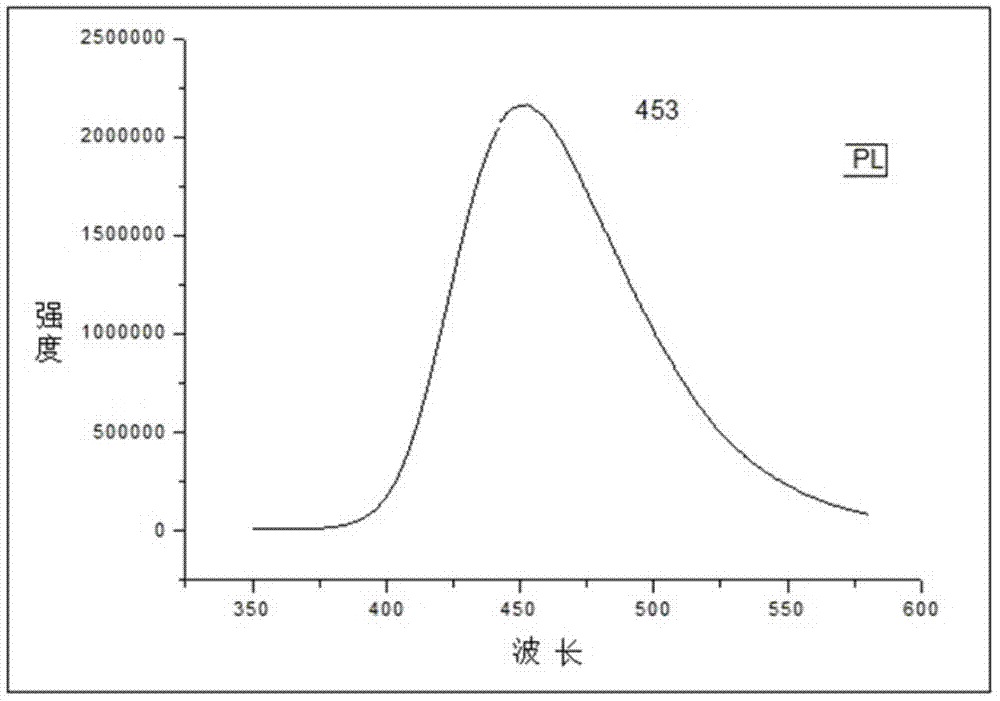
Indole derivative and application thereof to organic electroluminescence
A derivative, indole technology, applied to indole derivatives and their application in the field of organic electroluminescence display technology, can solve the problems of short lifespan of OLED devices, achieve high glass transition temperature and improve lifespan , the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of M1
[0070] The structural formula and synthetic route of the compound M1 to be prepared in this embodiment are as follows:
[0071] Synthesis of key intermediate M-1 (refer to the literature J.Org.Chem.2008,Vol.73,4638-4643 synthesis)
[0072]
[0073] Step A: In a 250ml three-neck flask, under the protection of nitrogen, add 4.71g (40.2mmol) of indole and 30ml of anhydrous DMF. Cool down to 0℃ in a cold bath, and add 1.95g (48.8mmol) of NaH (content 60%) in batches , Add 10ml of anhydrous DMF, keep at 0℃ and react for 15min, add 4.8ml (60.0mmol) of methyl iodide, slowly rise to room temperature, and react overnight (18h). Pour the reaction solution into a mixture of ethyl acetate and water, separate the layers, extract the aqueous phase with a small amount of ethyl acetate, combine the organic phases, dry with anhydrous MgSO4, filter and spin dry the filtrate to obtain an oil, which is subjected to column chromatography 5.51 g of yellow oily N-methylindole was...
Embodiment 2
[0079] Synthesis of M2
[0080] In this example, compound M2 is prepared, and its structural formula and synthetic route are as follows:
[0081]
[0082] The synthesis of intermediate M-2 is similar to that of M-1. In step A, ethyl iodide can be used instead of methyl iodide.
[0083] The synthesis of M2 adopts a method similar to that in Example 1, using an equivalent amount of 4-(9H-carbazol-9-yl)phenylboronic acid instead of 9-phenyl-9H-carbazole-3-boronic acid, and other conditions remain unchanged. Obtain M2 (white solid, yield 67%)
Embodiment 3
[0085] Synthesis of M3
[0086] In this example, compound M3 is prepared, and its structural formula and synthetic route are as follows:
[0087]
[0088] The synthesis of intermediate M-3 is similar to that of M-1, but in step A, iodobutane can be used instead of methyl iodide.
[0089] Synthesis of M3 adopts a method similar to that in Example 1, using an equivalent amount of triphenylamine 4-boronic acid instead of 9-phenyl-9H-carbazole-3-boronic acid, and other conditions remain unchanged to obtain M3 (white solid, yield 50 %)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com