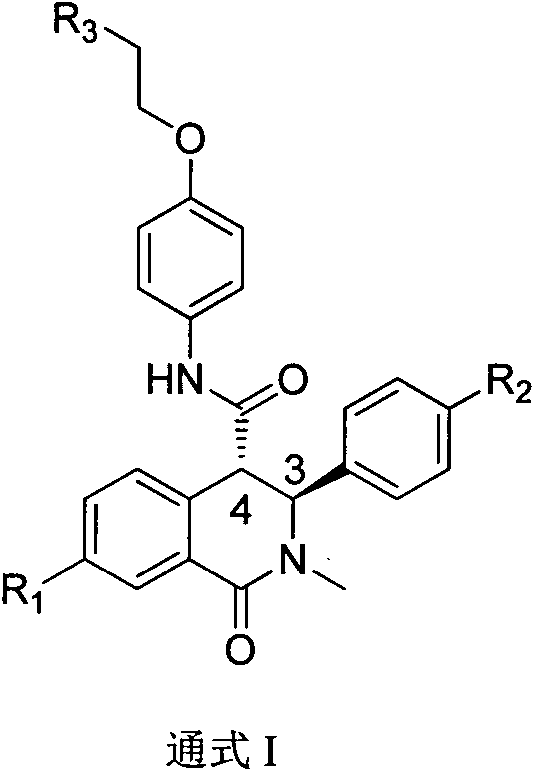
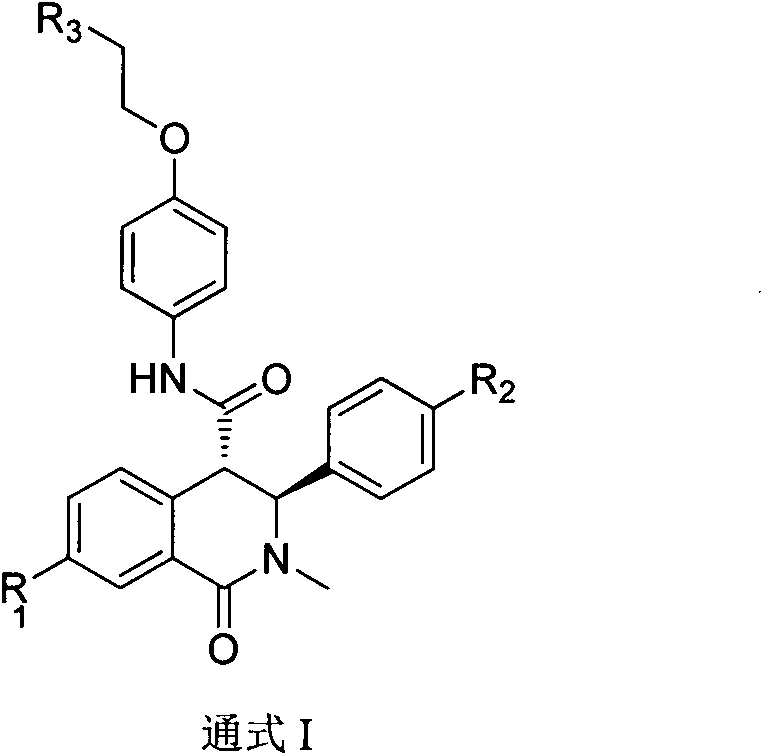
Synthetic method of trans-tetrahydroisoquinolone-4-carboxylic acid derivatives and medical application
A tetrahydropyrrole-based, pharmaceutical technology, used in drug combinations, anti-tumor drugs, organic chemistry, etc., can solve problems such as low toxicity and high efficiency, and no very satisfactory treatment methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Perphthalic Anhydride (II-1)
[0040] Dissolve 2.0g (11.1mmol) of perphthalic acid in 20ml of dry toluene, add 1.3ml (13.3mmol) of acetic anhydride dropwise, heat to reflux for 1 hour under the protection of nitrogen, and determine the end point by TLC. After the reaction solution is cooled, it is concentrated under reduced pressure to remove the solvent. 1.8 g of a light yellow solid was obtained with a yield of 99%. MS (ESI, m / z): 163 [M+H] + .
Embodiment 2
[0042] Synthesis of 4-methoxyperphthalic anhydride (II-2)
[0043] Dissolve 2.0g (9.5mmol) of 4-methoxyperphthalic acid in 20ml of dry toluene, add dropwise 1.1ml (11.4mmol) of acetic anhydride, heat and reflux for 1 hour under the protection of nitrogen, and determine the end point by TLC. Concentrate under reduced pressure to remove the solvent to obtain 1.7 g of light yellow solid with a yield of 99%. MS (ESI, m / z): 193 [M+H] + .
Embodiment 3
[0045] Synthesis of cis-N-methyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (IV-1)
[0046] Dissolve 5.0g (30.9mmol) of perphthalic anhydride II-1 in 60ml of acetonitrile, add 3.7g (30.9mmol) N-benzylidenemethylamine III-1 and 7.4g (15.5mmol) KA1(SO 4 ) 2 12H 2 O, react at room temperature for 8 hours, determine the end point by TLC, evaporate most of the solvent from the reaction solution under reduced pressure, add 100ml of water to the residue and stir for 10min, filter with suction, and wash the filter cake with methanol (25ml) and water (25ml) successively , 7.1 g of a white solid was obtained after drying, and the yield was 82%. 1 H-NMR (300MHz, DMSO-d6): δ12.24-12.25(s, 1H, 4-COOH), 6.54~7.42(m, 9H, Ar-H), 5.01~5.03(d, J=6.24Hz, 1H, 3-CH), 4.62~4.60 (d, J=6.24Hz, 1H, 4-CH), 2.90 (s, 3H, NCH 3 ); MS (ESI, m / z): 282 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com