A kind of method of producing dimethyl carbonate and ethylene glycol
A technology of dimethyl carbonate and ethylene glycol, which is applied in the preparation of organic carbonates, chemical instruments and methods, production of bulk chemicals, etc., can solve the problems of large equipment investment, low conversion rate of ethylene carbonate per pass, and cumbersome process flow, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
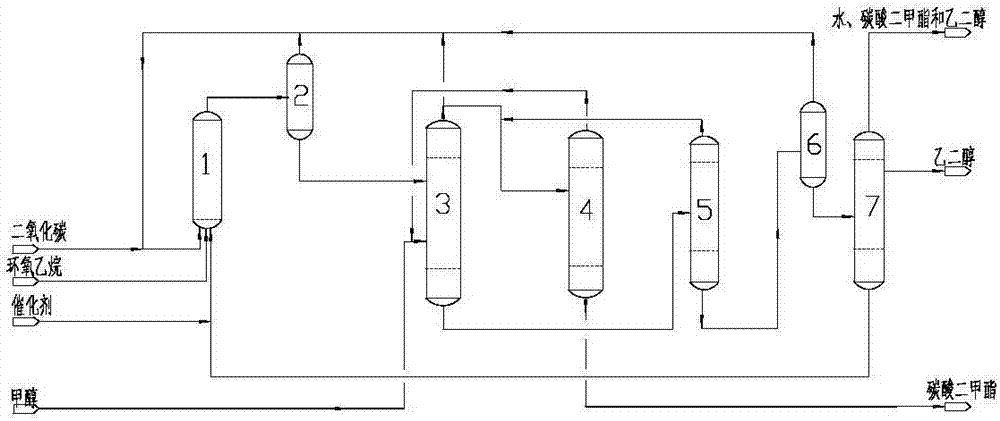
[0036] Referring to the figure, it is 0.5kg / h to produce dimethyl carbonate and ethylene glycol with the ethylene oxide feed flow as an example.
[0037] Raw material feed flow rate: ethylene oxide = 0.5 kg / h, carbon dioxide = 0.5 kg / h, methanol = 0.687 kg / h, water = 0.054 kg / h.
[0038] (1) Carbonylation step
[0039] Carbon dioxide and ethylene oxide react in the ethylene carbonate synthesis reactor 1 under the catalysis of the ionic liquid composite catalyst to generate ethylene carbonate. Theoretically, to generate 1kg of ethylene carbonate, it needs to consume 0.5kg of carbon dioxide and 0.5kg of ethylene oxide. In order to improve the conversion rate of ethylene oxide, it is necessary to increase the proportion of carbon dioxide. Excess carbon dioxide is recycled to the Ethylene carbonate synthesis reactor 1 inlet.
[0040] The operating pressure of ethylene carbonate synthesis reactor 1 is 1MPa, and the operating temperature is 190°C; the flow rates of carbon dioxide,...
Embodiment 2
[0058] Referring to the figure, the device for producing dimethyl carbonate and ethylene glycol at 0.5kg / h with ethylene oxide feed flow is an example.
[0059] Raw material feed flow rate: ethylene oxide = 0.5 kg / h, carbon dioxide = 0.5 kg / h, methanol = 0.721 kg / h, water = 0.003 kg / h.
[0060] (1) Carbonylation step
[0061] Carbon dioxide and ethylene oxide react in the ethylene carbonate synthesis reactor 1 under the catalysis of the ionic liquid composite catalyst to generate ethylene carbonate. Theoretically, 0.5 kg of carbon dioxide and 0.5 kg of ethylene oxide are required to generate 1 kg of ethylene carbonate. In order to increase the conversion rate of ethylene oxide, the proportion of carbon dioxide needs to be increased. Excess carbon dioxide is recycled to carbonic acid by flash tank and carbon dioxide compressor. Vinyl ester synthesis reactor 1 inlet.
[0062] The operating pressure of ethylene carbonate synthesis reactor 1 is 5MPa, and the operating temperatur...
Embodiment 3
[0080] Referring to the figure, the device for producing dimethyl carbonate and ethylene glycol at 0.5kg / h with ethylene oxide feed flow is an example.
[0081] Raw material feed flow rate: ethylene oxide = 0.5 kg / h, carbon dioxide = 0.5 kg / h, methanol = 0.714 kg / h, water = 0.018 kg / h.
[0082] (1) Carbonylation step
[0083] Carbon dioxide and ethylene oxide react in the ethylene carbonate synthesis reactor 1 under the catalysis of the ionic liquid composite catalyst to generate ethylene carbonate. Theoretically, 0.5 kg of carbon dioxide and 0.5 kg of ethylene oxide are required to generate 1 kg of ethylene carbonate. In order to increase the conversion rate of ethylene oxide, the proportion of carbon dioxide needs to be increased. Excess carbon dioxide is recycled to carbonic acid by flash tank and carbon dioxide compressor. Vinyl ester synthesis reactor 1 inlet.
[0084] Ethylene carbonate synthesis reactor 1 operating pressure is 5MPa, operating temperature is 180 ℃; Eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com