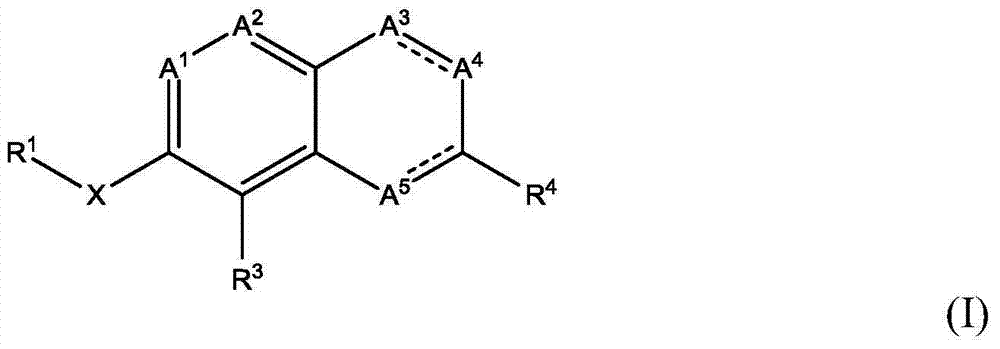
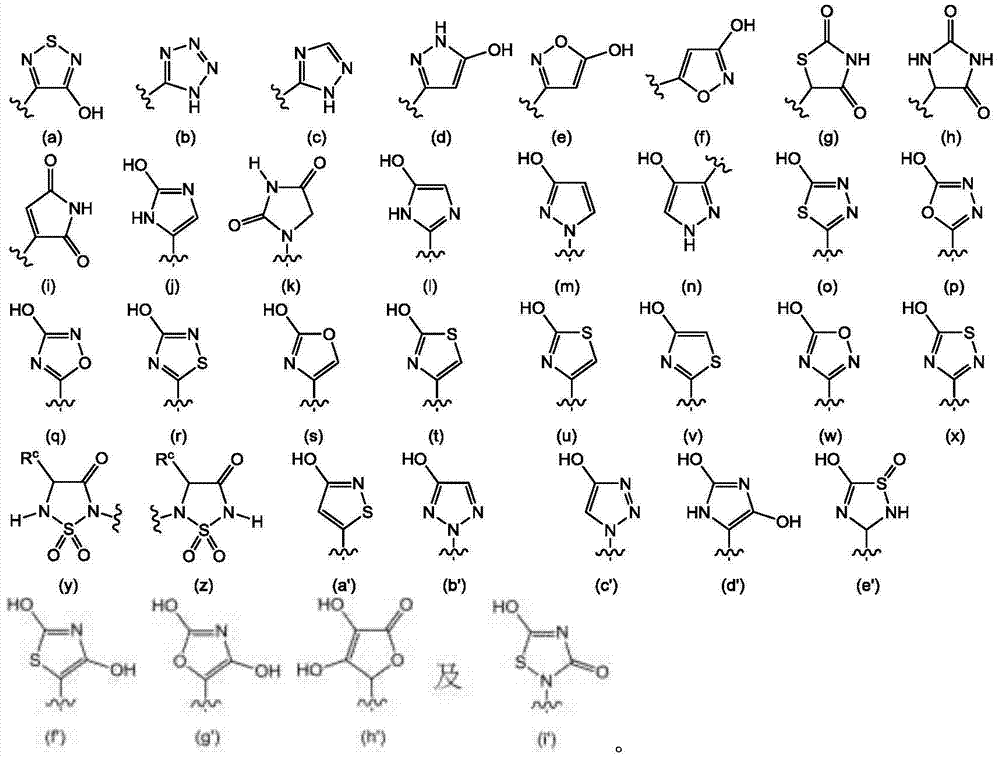
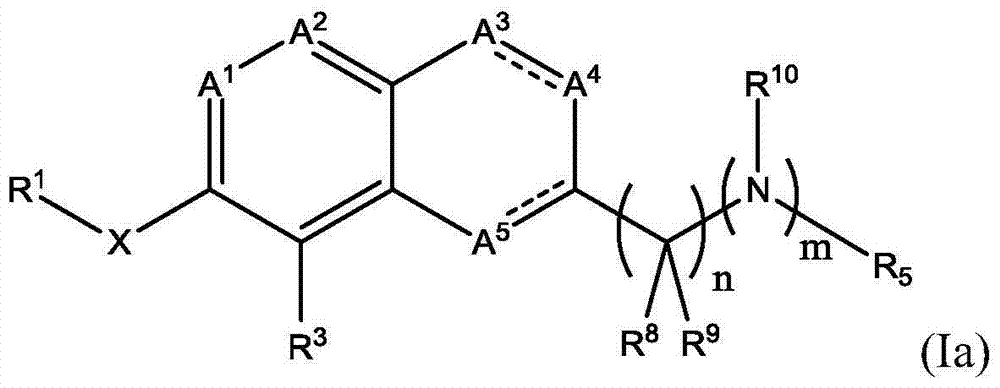
Atx modulating agents
A kind of alkyl, independent technology, application in the field of ATX modulator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0691] Example 1: 4-((7-((cis-4-methylcyclohexyl)oxy)-8-(trifluoromethyl)naphthalen-2-yl)methyl)morpholine
[0692]
[0693] Step 1: 2-Bromo-7-(cis-4-methyl-cyclohexyloxy)-naphthalene
[0694]
[0695] To a mixture of 2-bromo-7-hydroxynaphthalene (1.2 g, 0.0053 mol) and cesium carbonate (3.440 g, 0.01056 mol) in N,N-dimethylformamide (10 mL, 0.1 mol) was added in two portions cis-4-methyl-cyclohexyl methanesulfonate (2 g, 0.01 mol). The resulting mixture was heated at 85 °C overnight, and cooled to room temperature, and washed with Et 2 O diluted, washed with water, brine and washed with Na 2 SO 4 dry. The crude mixture was then purified by silica gel (EtOAc / heptane gradient, 0% to 30%) to give the product 2-bromo-7-(cis-4-methyl-cyclohexyloxy)-naphthalene as a solid (778 mg, 46%). LCMS RT = 2.53 min, m / z = 319.10 [M+]. 1H NMR (400MHz, chloroform-d) δ7.86(s, 1H), 7.71(d, J=8.97Hz, 1H), 7.62(d, J=8.66Hz, 1H), 7.38(d, J=8.66Hz ,1H),7.17(d,J=8.85Hz,1H),7.06(s,1H),4...
Embodiment 2
[0714] Example 2: 9-[7-(cis-4-methyl-cyclohexyloxy)-8-trifluoromethyl-naphthalen-2-ylmethyl]-9-aza-bicyclo[3.3.1] Nonane-3-carboxylic acid
[0715]
[0716] 7-(cis-4-methyl-cyclohexyloxy)-8-trifluoromethyl-naphthalen-2-ylmethyl methanesulfonate (105mg, 0.252mmol) in N,N-dimethylformamide (2.9 mL, 38 mmol) was added methyl 9-aza-bicyclo[3.3.1]nonane-3-carboxylate HCl salt (110.79 mg, 0.50426 mmol) followed by cesium carbonate (246.44 mg, 0.75638 mmol). The reaction was then heated at 80°C for 1 hour. LCMS showed no SM remaining and the reaction was complete (RT 1.60 min; MH+504.3). After cooling, the reaction mixture was diluted with EtOAc and washed with water (2x). The organic phase is then separated, dried and concentrated. The crude material was purified by HPLC, the solvent was removed, and the ester was dissolved in tetrahydrofuran (1.2 mL, 14 mmol) and treated with 1.0 M lithium hydroxide in water (1.8 mL, 1.8 mmol) overnight at room temperature. Acidified with ...
Embodiment 3
[0717] Example 3: 8-[7-(cis-4-methyl-cyclohexyloxy)-8-trifluoromethyl-naphthalen-2-ylmethyl]-8-aza-bicyclo[3.2.1] Octane-3-carboxylic acid
[0718]
[0719] 7-(cis-4-methyl-cyclohexyloxy)-8-trifluoromethyl-naphthalen-2-ylmethyl methanesulfonate (105mg, 0.252mmol) in N,N-dimethylformamide 8-Aza-bicyclo[3.2.1]octane-3-carboxylic acid methyl ester HCl salt (103.72mg, 0.50426mmol) and cesium carbonate (246.44mg, 0.75638mmol) were added sequentially to a solution in (2.9mL, 38mmol) . The reaction was then heated at 80°C for 1 hour. LCMS showed no SM remaining and the reaction was complete (RT 1.56 min; MH+ 490.3 and 1.49 min, 476.30). After cooling, the reaction mixture was diluted with EtOAc and washed with water (2x). The organic phase is then separated, dried and concentrated. The crude material was purified by HPLC, the solvent was removed, and the ester was dissolved in tetrahydrofuran (1.2 mL, 14 mmol) and treated with 1.0 M lithium hydroxide in water (1.8 mL, 1.8 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com