B crystal form of empagliflozin and preparation of B crystal form
A technology of empagliflozin and crystal form, applied in the direction of organic chemistry, organic chemical methods, etc., can solve the problems of unsatisfactory, reduced impurity content, compound purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 Empagliflozin crude product
[0033] (1) chemical reaction formula
[0034]
[0035] (2) Operation process
[0036] In a 50L reaction kettle, dissolve 3.4kg (5.5mol) of the acetyl-protected product VI in 17L of acetonitrile, stir at room temperature to form a milky (yellow) white suspension, and add 10% NaOH to the constant pressure dropping funnel Aqueous solution 1.1kg. Heat to 60°C and stir for about 1 hour, the system gradually dissolves, and after the reaction is complete as monitored by HPLC, cool down to room temperature. Add about 5L of concentrated HCl to adjust the pH value to neutral, stir and crystallize overnight. After filtration, about 1.20 kg of a light yellow-white product can be obtained, and the purity detected by HPLC is about 98%. MASS: 451 [M+H]+. Used in the preparation of Empagliflozin B crystal form in each of the following examples.
Embodiment 2
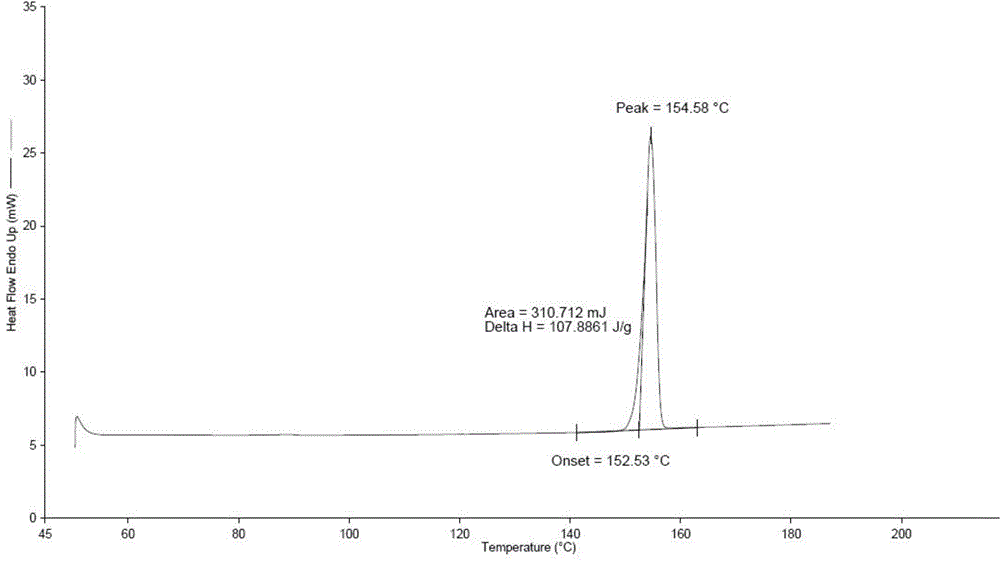
[0037] The preparation of embodiment 2 empagliflozin crystal form B
[0038] In the 20L crystallization tank, add the empagliflozin crude product of the gained embodiment 1 of 1kg, add the methyl ethyl ketone that mass ratio is 1:1: each 5kg of water, be down to room temperature (control cooling rate is 1 ℃) after heating up and refluxing to dissolve clear / min), stirred and crystallized for 30min, filtered to obtain a product with a wet weight of about 900g of about 99.7% purity. After 16 hours in a vacuum oven at 40°C, 803 g of empagliflozin was obtained, with a yield of 80.3%. Purity by HPLC: 99.89%. MASS: 451 [M+H]+.
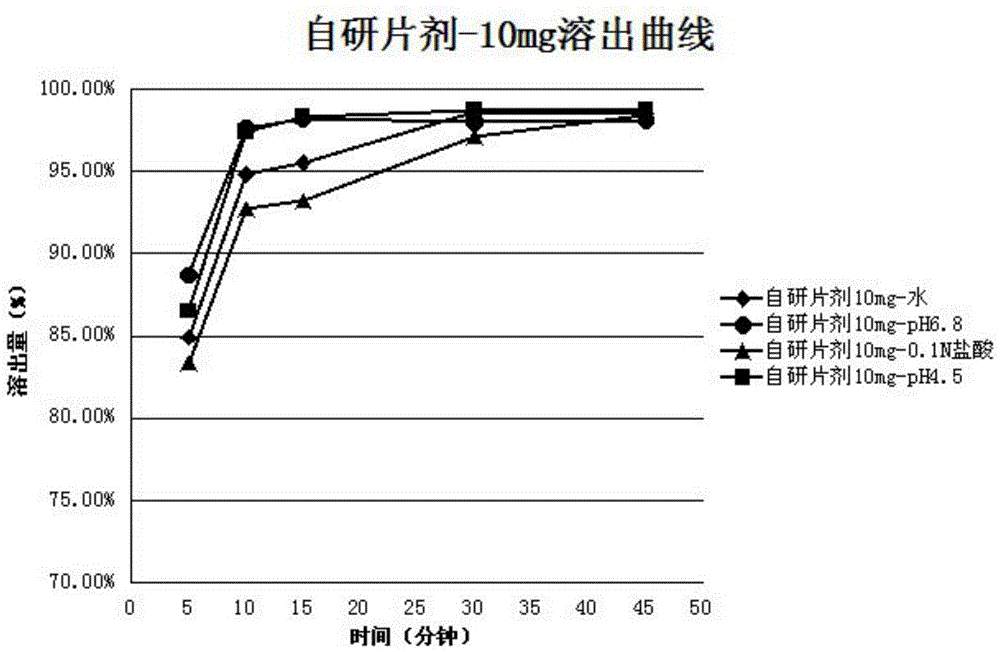
[0039] It has been proved by experiments that when the purity of the empagliflozin raw material used to prepare the crystal form B is above 97.5%, the crystal form B of empagliflozin with a purity of above 99.8% can be obtained. During the preparation process, if it is necessary to obtain Empagliflozin Form B with higher purity, the recrystallization meth...
Embodiment 3
[0040] Embodiment 3 Empagliflozin crystal form B stability research
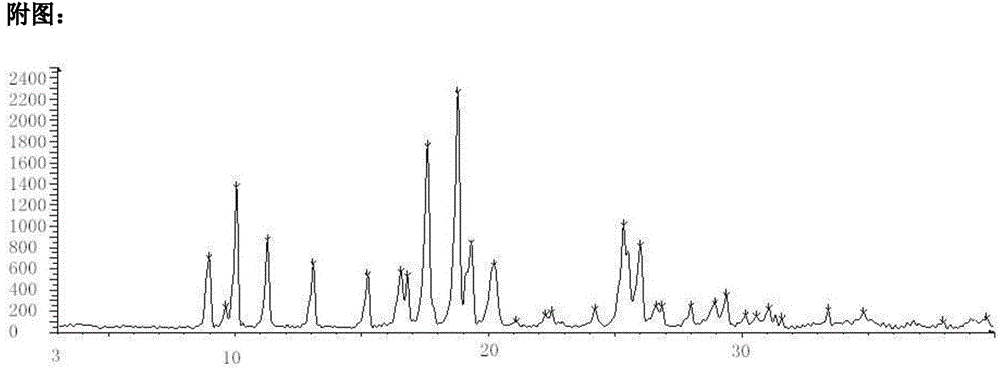
[0041] Empagliflozin crystal form B has been carried out stability study: [high temperature (60 ℃), high humidity (90% ± 5%), light (4500lx)], accelerated test (temperature 40 ℃ ± 2 ℃, relative humidity 75 %±5%) and long-term test (temperature 25°C±2°C, relative humidity 60%±10%) and X-ray powder diffraction test on the sample after grinding and tableting, and purity test by HPLC method, test The result looks like this:
[0042]
[0043] The test results show that: after grinding and tableting, the main 2θ angles of this product have not changed significantly by X-ray powder diffraction test, indicating that the crystal form of this product has good stability during the preparation process, and the purity detected by HPLC has not changed significantly. Changes, excellent chemical stability. In addition, after 6 months of accelerated testing and 12 months of long-term sample retention for the raw materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com